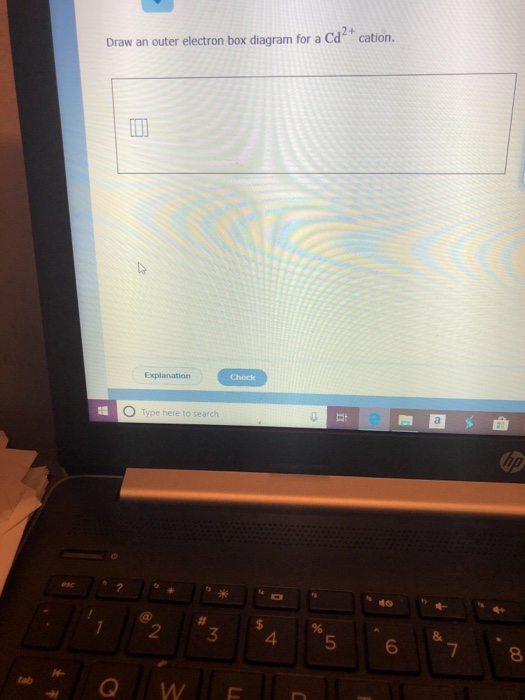
37 draw an outer electron box diagram for a cation.
The Lewis structure and electron-domain geometry of SF. 4. are shown in Sample Exercise 9.2. The S atom has five electron domains around it, giving rise to a trigonal -bipyramidal electron -domain geometry. With an expanded octet of ten electrons, a . d . orbital on the sulfur must be used. The trigonal -bipyramidal electron-domain geometry ... Draw schematic diagrams for the electrons in the subshells of (a) sodium (Na) and (b) argon (Ar) atoms in the ground state. Book Cover for College Physics 7th ...
Draw an outer electron box diagram for a CO^2+ cation. Question: Draw an outer electron box diagram for a CO^2+ cation. This problem has been solved! ... Draw an outer electron box diagram for a CO^2+ cation. Previous question Next question. COMPANY. About Chegg; Chegg For Good; College Marketing;

Draw an outer electron box diagram for a cation.
For orbital diagrams, this means two arrows go in each box (representing two electrons in each orbital) and the arrows must point in opposite directions (representing paired spins). The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. Refer to the explanation. The electron configuration of manganese, atomic number 25, is "1s"^2"2"^2"2p"^6"3s"^2"3p"^6"3d"^5"4s"^2". The diagram below represents the electron configuration as an orbital diagram. In order to write the Iron electron configuration we first need to know the number of electrons for the Fe atom (there are 26 electrons). Once we have the configuration for Fe, the ions are simple. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the Iron atom. Video: Fe, Fe2+, and Fe3+ Electron ...
Draw an outer electron box diagram for a cation.. Valence calculator The electronic configuration of many ions is that of the closest noble gas to ... optimal state of having a full valence (outermost) shell of electrons. A good place to start when trying to figure out the electron configuration of an ion is the electron configuration of the neutral parent atom.. In this case, titanium, #"Ti"#, is located in period 4, group 4 of the periodic table and has an atomic number of #22#. This means that a neutral titanium atom will contain #22# protons in its nucleus and #22# electrons surrounding its nucleus. Science. Chemistry. Chemistry questions and answers. 4+ Draw an outer electron box diagram for a Cr* cation.
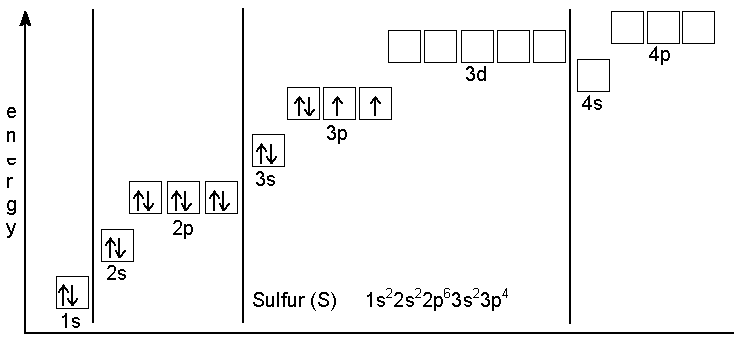
Draw the complete electron box diagram for the following atoms a) Carbon Sulfur Calcium Molybdenum e) Tin 9) Draw the complete electron box diagrams for the following ions_ Fluoride. View Full Video. Already have an account? Log in Matthew H. University of Delaware. Answer. Model Ill: Complex Lewis Dot Structures / Polyatomic Ions This! Use the atom cards and Cheerios to build the polyatomic ion before drawing it. Polyatomic ions are a group of atoms that are covalently bonded and act as a single unit with a charge. Building Lewis dot structures for polyatomic ions follows the same process as building a molecule. Electron configuration for Ru3+. Electron configuration for Ru 3+. What scientific concept do you need to know in order to solve this problem? Our tutors have indicated that to solve this problem you will need to apply the The Electron Configuration: Ions concept. You can view video lessons to learn The Electron Configuration: Ions. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
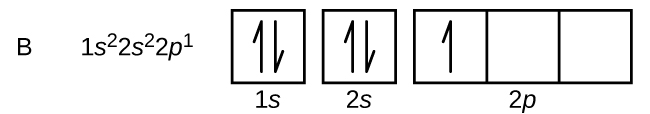
After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4. Therefore the expected electron configuration for Chromium will be 1s 2 2s 2 2p 6 3s 2 3p 4 4s 2 3d 9. Note that when writing the electron configuration for an atom like Cr, the 3d is usually written before the 4s. 1. Draw an "electron dot" diagram showing the first 18 elements in the periodic table. 2. Explain how the electron dot diagram is similar for families in the periodic table. 3. Draw an electron dot diagram showing the formation of ions and ionic compounds. 4. Explain how hydrogen can be considered as behaving like a metal or a nonmetal. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... Give the electron configuration for nitrogen (N) and draw an Aufbau diagram. ... Electrons in the outermost energy level of an atom are called valence ...
Therefore the Calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Video: Calcium Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Therefore the Potassium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1. Video: Potassium Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom.
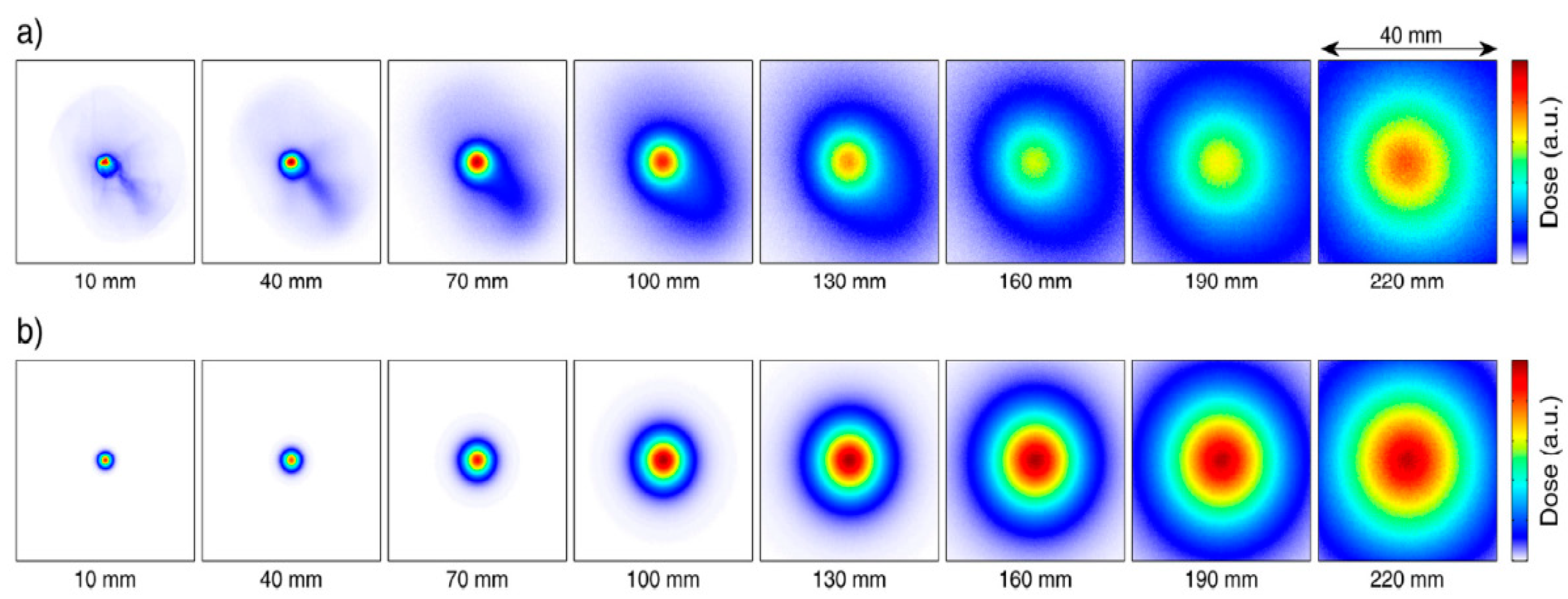
Applied Sciences Free Full Text Towards Laser Driven Hadron Cancer Radiotherapy A Review Of Progress Html
When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. In this figure, the element symbol ...
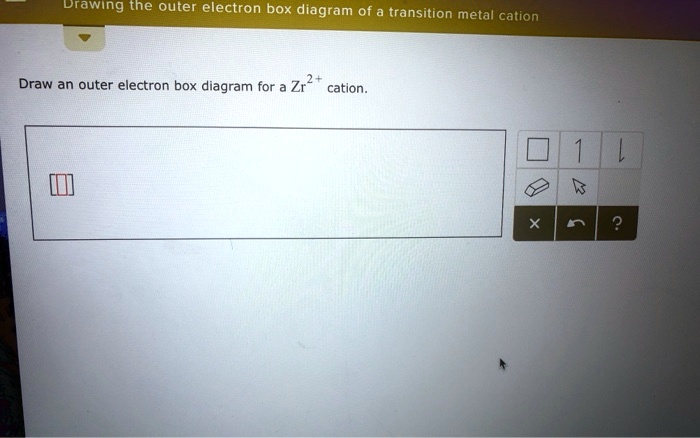
Solved Drawing The Outer Electron Box Diagram Of Transition Metal Cation Draw An Outer Electron Box Diagram For Zr2 Cation
This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry.
Question: 4+ Draw an outer electron box diagram for a Nb" cation. Kr 4d 5s . This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text ... 4+ Draw an outer electron box diagram for a Nb" cation. Kr 4d 5s . Previous question Next question. COMPANY. About Chegg; Chegg For Good; College ...
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Rh2+ have 43 electrons. It's …. View the full answer. Transcribed image text: Draw an outer electron box diagram for a Rh cation.
Draw an outer electron box diagram for a {eq}Mo^{2+}{/eq} cation. Transition Elements In the periodic table, elements are classified on the basis of their electronic configuration meaning the ...
For the sodium phosphate example, we can build this molecule using the same charge box diagram that we used above to construct the simpler biatomic structures above. First we need to place the ions and their charge states into the table. In this case, we know that sodium is a cation with a +1 charge and the phosphate ion is an anion with a -3 charge.
["Kr"]4d^10 Your starting point here will be the electron configuration of a neutral cadmium atom. Cadmium, "Cd", is located in period 5, group 12 of the periodic table and has an atomic number equal to 48. This means that a neutral cadmium atom will have a total of 48 electrons surrounding its nucleus. This also tells you that the "Cd"^(2+) cation, which has two electrons less than the ...
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Ground state election configuration of Tc = [Kr] 4d5 5s2 S …. View the full answer. Transcribed image text: Draw an outer electron box diagram for a Tc cation. ク.
Using outer electron shells only, draw 'dot-and-cross' diagrams for molecules of BF3 and NH3. Use your diagrams to explain why a molecule of BF3 has bond angles of 120° and NH3 has bond angles of 107°.
(5 points) (a) Write the short-hand electron configuration of Vanadium. (b) Draw the orbital diagram of the valence electrons in Vanadium. (c) Write the full ...
Question: Draw a dot- and- cross diagram for magnesium fluoride, showing all electrons. Answer: Dot- and- cross diagram for ionic compound -- magnesium fluoride. Part 3: Dot- and- cross diagrams for simple molecules (covalent molecules). Recap - Covalent molecules are made up of two of more atoms of non- metals.
Orbital diagrams make use of a box, circle, or line for each ... same outer electron configuration. Elements in the same group of the periodic table exhibit ... gaseous atoms or ions. Atoms with a . low IE. tend to form . cations. Atoms with a . high IE. tend to form . anions (except the
When the electron gets excited, the angular momentum 0.529 =4 0.132 Since the atomic number is 4, the element is beryllium. = 4.20 × 10–34 joules. 1 1 E3 − E2 = −21.72 × 10 −19 − 9 4 An electron can lose energy when it is present in the 4th orbit and not from the 1st orbit. 5 36
The most important characteristic of electron in the production of X-rays is (a) charge of electron (b) mass of electron (c) revolution of electron around the nucleus (d) speed of electron 44. 450 (a) (c) The energy of a photon of light of wavelength nm is 4.4 x 10"' 9 J (b) 2.5 x l o M 9 J 17 K25 x 10~ J (d) 2.5 x 10-'7 J 45. A positron has the same mass as (a) proton (b) a-particle (c ...
To write the configuration for the Cobalt ions, first we need to write the electron configuration for just Cobalt (Co). We first need to find the number of ...

1 Write Orbital Diagrams For Each Of These Ions A V5 B Cr3 C Ni2 D Fe3 2 Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2
Once we have the configuration for Cu, the ions are simple. When we write the configuration we'll put all 29 electrons in orbitals around the nucleus of the ...
Enter the email address you signed up with and we'll email you a reset link.
representing electron density that surrounds both cations and anions. D In electron density maps for ionic compounds, ... Draw dot and cross diagrams for the lithium and iodide ions. Show all the electrons in the lithium ion but only outer shell electrons in the iodide ion. (2) (b) On the Born-Haber cycle below, fill in the missing formulae ...
How to draw bohr diagrams michelle bartels bohr diagrams 1 draw a nucleus with the number of protons and neutrons inside 2 carbon is in the 2nd period so it has two energy levels or shells. This lesson will walk your students through the basics on how to draw a bohr diagram for the first 20 elements on the periodic table. 2 carbon has 6 ...
Atkins Physical Chemistry 10th Solutions - Free ebook download as PDF File (.pdf), Text File (.txt) or read book online for free. physical chemistry
Answer to: Draw an outer electron box diagram for a Nb^{3+} cation. By signing up, you'll get thousands of step-by-step solutions to your...
1.6.1c draw electron configuration diagrams of cations and anions using dots or crosses to represent electrons AND 1.6.2b draw electron configuration diagrams for simple covalently bonded molecules, including those with multiple bonds and dative covalent bonds, using dots or crosses to represent electrons
In order to write the Iron electron configuration we first need to know the number of electrons for the Fe atom (there are 26 electrons). Once we have the configuration for Fe, the ions are simple. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the Iron atom. Video: Fe, Fe2+, and Fe3+ Electron ...

A Level Chemistry 1 6 1c Draw Electron Configuration Diagrams Of Cations And Anions Using Dots Or Crosses To Represent Electrons And 1 6 2b Draw Electron Configuration Diagrams For Simple Covalently Bonded Molecules Including Those With
Refer to the explanation. The electron configuration of manganese, atomic number 25, is "1s"^2"2"^2"2p"^6"3s"^2"3p"^6"3d"^5"4s"^2". The diagram below represents the electron configuration as an orbital diagram.

A Level Chemistry 1 6 1c Draw Electron Configuration Diagrams Of Cations And Anions Using Dots Or Crosses To Represent Electrons And 1 6 2b Draw Electron Configuration Diagrams For Simple Covalently Bonded Molecules Including Those With
For orbital diagrams, this means two arrows go in each box (representing two electrons in each orbital) and the arrows must point in opposite directions (representing paired spins). The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom.


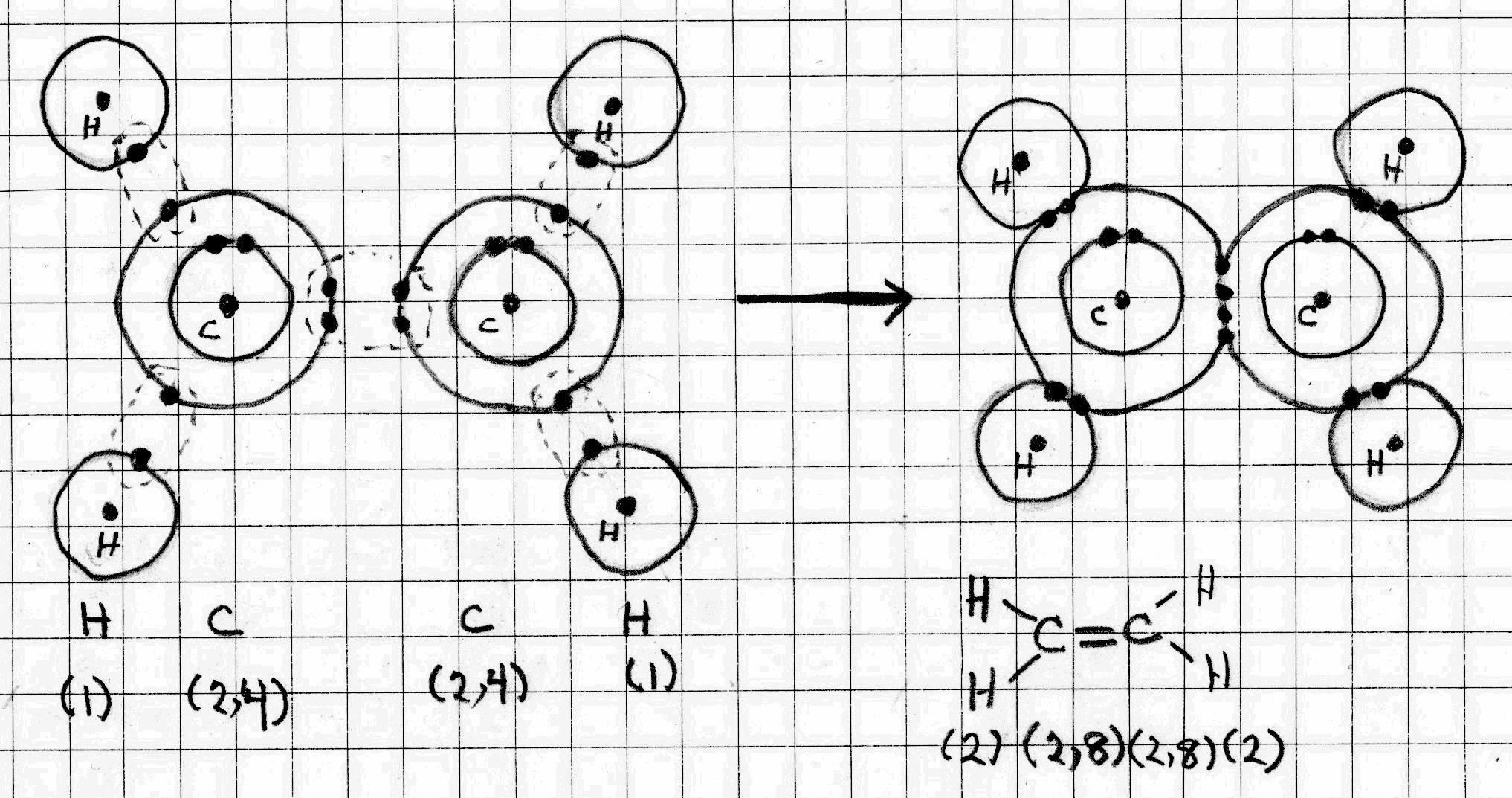
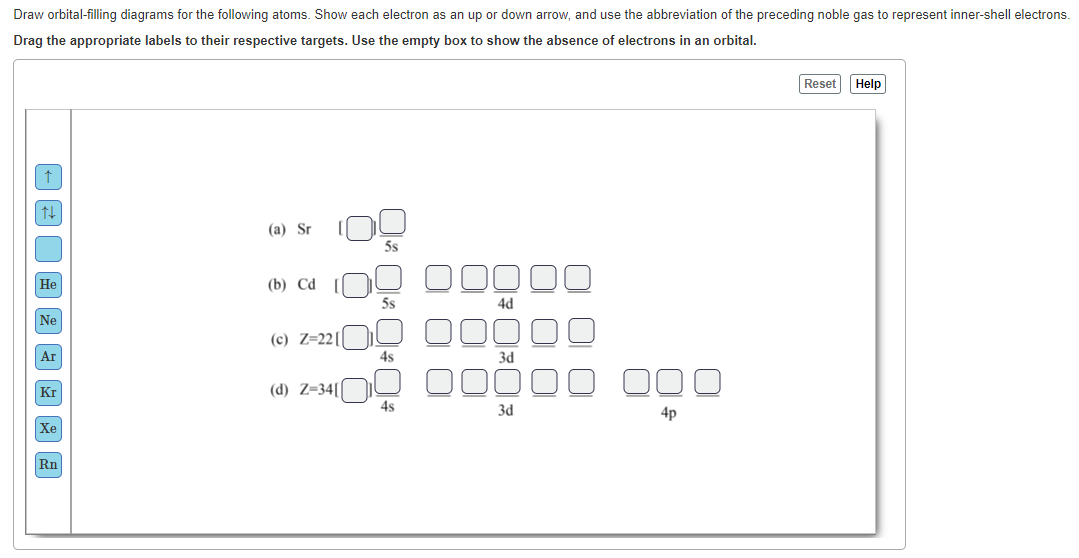














0 Response to "37 draw an outer electron box diagram for a cation."
Post a Comment