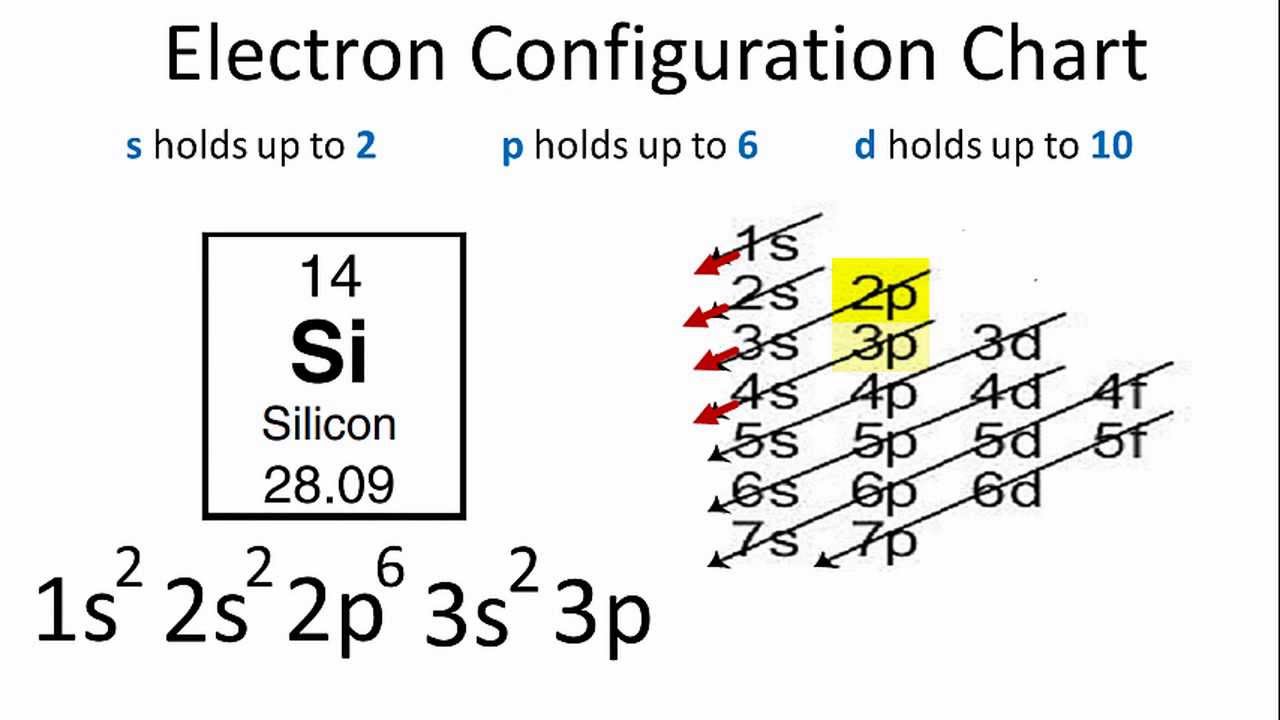
37 lewis dot diagram for silicon
Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1. Construct the Lewis structure for the covalent compound silicon dioxide (SiO 2). Learn this topic by watching Lewis Dot Structures: Neutral Compounds Concept Videos All Chemistry Practice Problems Lewis Dot Structures: Neutral Compounds Practice Problems
Lewis Dot Structure of Atoms Link: Determining Shape Video: ... Lewis Structure: Silicon Hexafluoride(2-) Ion: SiF 6 2-Lewis Structure: Triodide(-) Ion: I 3-Lewis Structure . Main Group Acids, Anions and Oxygen Compounds: Chlorine Dioxide: ... Lewis Structure: My Chemical Demonstration Videos ...

Lewis dot diagram for silicon
Below is the image of the lewis dot structure of Silicon and Sulfur separately. Now let us study the steps involved to draw the Lewis structure of Silicon disulfide (SiS2): Step 1 : Note down the total number of valence electrons available to draw one molecule of silicon disulfide : It is 16 as 4 are coming from silicon atom and 6 are coming ... SiO2 Lewis Structure (Step by Step Construction) In the SiO 2 lewis Structure, the overall ratio of silicon to the oxygen atom is 1:2. The Silicon oxygen bonds are strong and keep the atoms firmly in place. Following are the steps to construct the SiO 2 Lewis Structure. Step-1: Count the valence electrons of atoms. Transcribed image text: Some Scientists think that life elsewhere in the universe might be based on the element silicon, rather than on carbon, as on Earth. Look at the electron distribution diagram for silicon, and draw the Lewis dot structure for silicon on your note. Analyze your knowledge about it and propose a short essay about the possible silicon-based life.
Lewis dot diagram for silicon. Silicon tetraiodide is the chemical compound with the formula Si I 4. It is a tetrahedral molecule with Si-I bond lengths of 2.432 (5) Å. SiI 4 is a precursor to silicon amides of the formula Si (NR 2) 4 (R = alkyl). It has also been of interest in the manufacture and etching of silicon in microelectronics . How to draw lewis structure for sih4. Chemistry learning made easy. This video shows you how to draw the lewis dot diagram structure for sih4 silicon tetrahydride. It provides molecular geometry, bond angle, and hybridi. A stepbystep explanation of how to draw the sih4 lewis structure (silicon tetrahydride). Both use all 20 valence el. A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure (Silicon Tetrahydride).For the SiH4 structure use the periodic table to find the total... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
The lewis dot structure ignores the nucleus and all non-valence electrons, displaying only the valence electrons of an atom. If the atom is a noble-gas atom, two alternative procedures are possible. Either we can consider the atom to have zero valence electrons or we can regard the outermost filled shell as the valence shell. Answer (1 of 2): Apparently there are different schools of thought on this (How to Draw Electron Dot Diagrams). That said, it seems that the one followed (at least in the Western US) has you start with the right hand side and the S orbital (only the outer valence electrons!): Si: (easier to do t... Silicon Dioxide (SiO2) Lewis Structure. The Lewis structure of SiO2 is identical to the Lewis structure of CO2. The only difference is that instead of carbon, silicon is used. One silicon atom is at the middle, with two oxygen atoms bound to it in a double bond. There are no lone pairs on the central atom of the SiO2 Lewis dot structure, The electron and molecular geometry of SiO2 are linear. The bond angle of Silicon dioxide is 180º and the hybridization of it is Sp. The total valence electron available for the Silicon dioxide lewis structure is 16. The formal charge in the SiO2 lewis dot structure is zero. SiO2 is a non-polar molecule.
The Lewis structure for silicon disulfide is to be predicted. Concept introduction: The strategy for drawing Lewis structure is mention below. Calculate the number of valence electrons present in the molecule. Calculate the electron pairs by diving number of valence electrons by 2. Determine the bond pairs. Determine the lone pairs. A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. When constructing a Lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share electrons until they are surrounded by eight ... Laboratory Chemical Safety Summary (LCSS) Datasheet. Molecular Formula. S2Si. Synonyms. Silicon disulfide. 13759-10-9. Silicon sulfide (SiS2) bis (sulfanylidene)silane. UNII-35Y5PHW16K. GHS Hazard Statements: H301 (100%): Toxic if swallowed [Danger Acute toxicity, oral]H311 (90.7%): Toxic in contact with skin [Danger Acute toxicity, dermal]H314 (100%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation]H317 (90.7%): May cause an allergic skin reaction [Warning Sensitization, Skin]H334 (90.7%): May cause allergy or asthma symptoms or breathing ...
atoms. We do this by forming what are called Lewis diagrams. In Lewis diagrams the atoms are shown by writing the atomic symbol surrounded by one dot for each of the valence electrons. In a covalently bound molecule the dots are arranged in pairs, with the bound pairs placed between the atoms which they connect
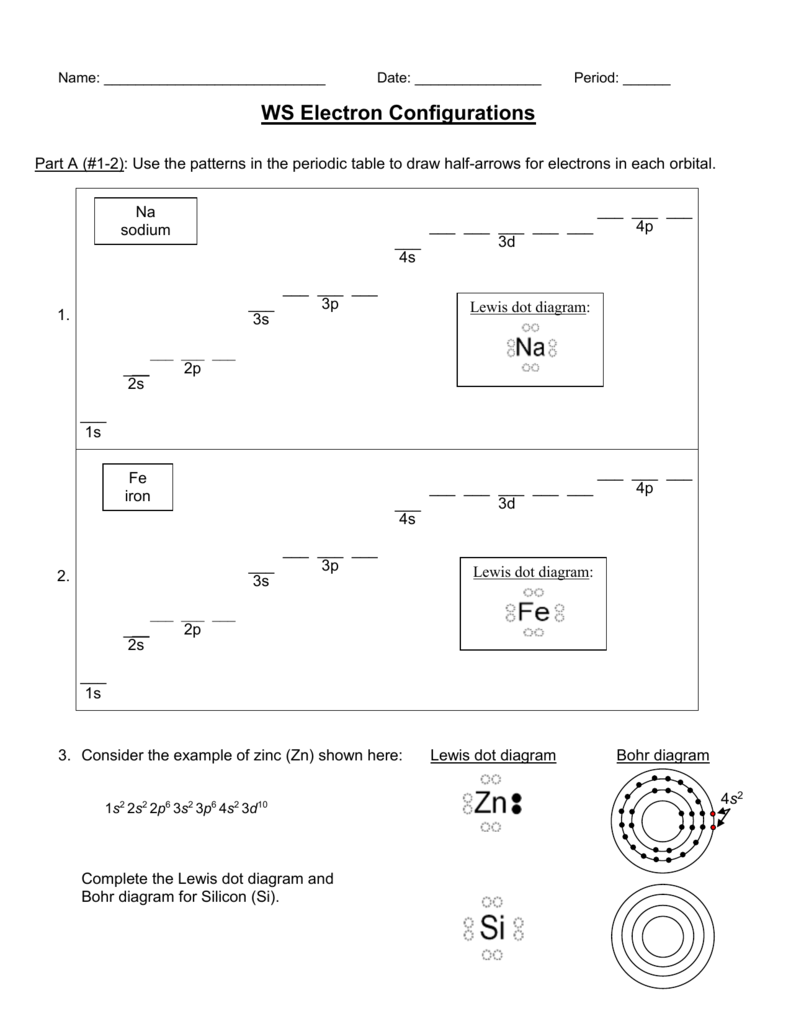
9. Draw a Lewis dot diagram for the barium atom. 10. Draw the Lewis dot diagram for the silicon atom. I I, Draw the Lewis dot diagram for the iodine atom. 12. Draw the Lewis dot diagram for the xenon atom. 13. Hypothesize: Why are noble gases considered to be non-reactive? Your group will check your answers with the instructor before moving on.
For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4s 2 3d 6) is as follows:. Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration.
Write the electron dot (Lewis) diagrams for the following. 9. carbon silicon Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. Lewis structures extend the ...
XeO 2 F 2. Steps for Writing Lewis Structures. Find the total valence electrons for the molecule. Explain How Examples: H 2 S, NCl 3, OH -. Put the least electronegative atom in the center. Note: H always goes outside. Examples: NOCl, CF 2 Cl 2, HCN. Put two electrons between atoms to form a chemical bond.
What element has the same Lewis dot structure as silicon? No. What is the lewis dot diagram for carbon dioxide? Refer to the related link for an illustration of the Lewis dot diagram for carbon ...
Silicon is in group 14 and period 3 of the periodic table and has four valence electrons in its Lewis structure. The four valence electrons means that silicon can bond in a way similar to carbon.
Lewis Structure: A Lewis structure is a diagram that describes the covalent bonding between two or more atoms present in a molecule or ion. The atoms are written in terms of their elemental symbols.
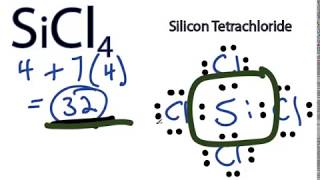
Follow some steps for drawing the lewis dot structure of SiCl4. 1. Count total valence electron in SiCl4. First of all, find the availability of valence electrons for SiCl4, for this, just find the valence electron of silicon and chlorine atoms through the periodic group number.
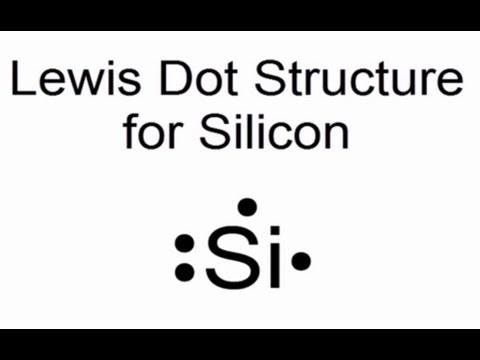
A step-by-step explanation of how to draw the Lewis dot structure for Si (Silicon). I show you where Silicon is on the periodic table and how to determine h...
Bohr Model of Silicon
Transcribed image text: Some Scientists think that life elsewhere in the universe might be based on the element silicon, rather than on carbon, as on Earth. Look at the electron distribution diagram for silicon, and draw the Lewis dot structure for silicon on your note. Analyze your knowledge about it and propose a short essay about the possible silicon-based life.
SiO2 Lewis Structure (Step by Step Construction) In the SiO 2 lewis Structure, the overall ratio of silicon to the oxygen atom is 1:2. The Silicon oxygen bonds are strong and keep the atoms firmly in place. Following are the steps to construct the SiO 2 Lewis Structure. Step-1: Count the valence electrons of atoms.
Below is the image of the lewis dot structure of Silicon and Sulfur separately. Now let us study the steps involved to draw the Lewis structure of Silicon disulfide (SiS2): Step 1 : Note down the total number of valence electrons available to draw one molecule of silicon disulfide : It is 16 as 4 are coming from silicon atom and 6 are coming ...

Draw The Lewis Structure For Sicl4 How Many Bonds Are Around The Central Atom And What Is The Shape Of This Molecule Study Com

Solved 9 1 Draw The Lewis Lectron Dot Diagram For Each Element Strontium Silicon Krypton Sulfur 9 2 Draw The Lewis Lectron Dot Diagram For Each Ion 9 3 How Many Electrons Does Pb Atom Have

Construct The Lewis Structure For The Covalent Compound Silicon Dioxide Sio2 Using The Following Steps Home Work Help Learn Cbse Forum









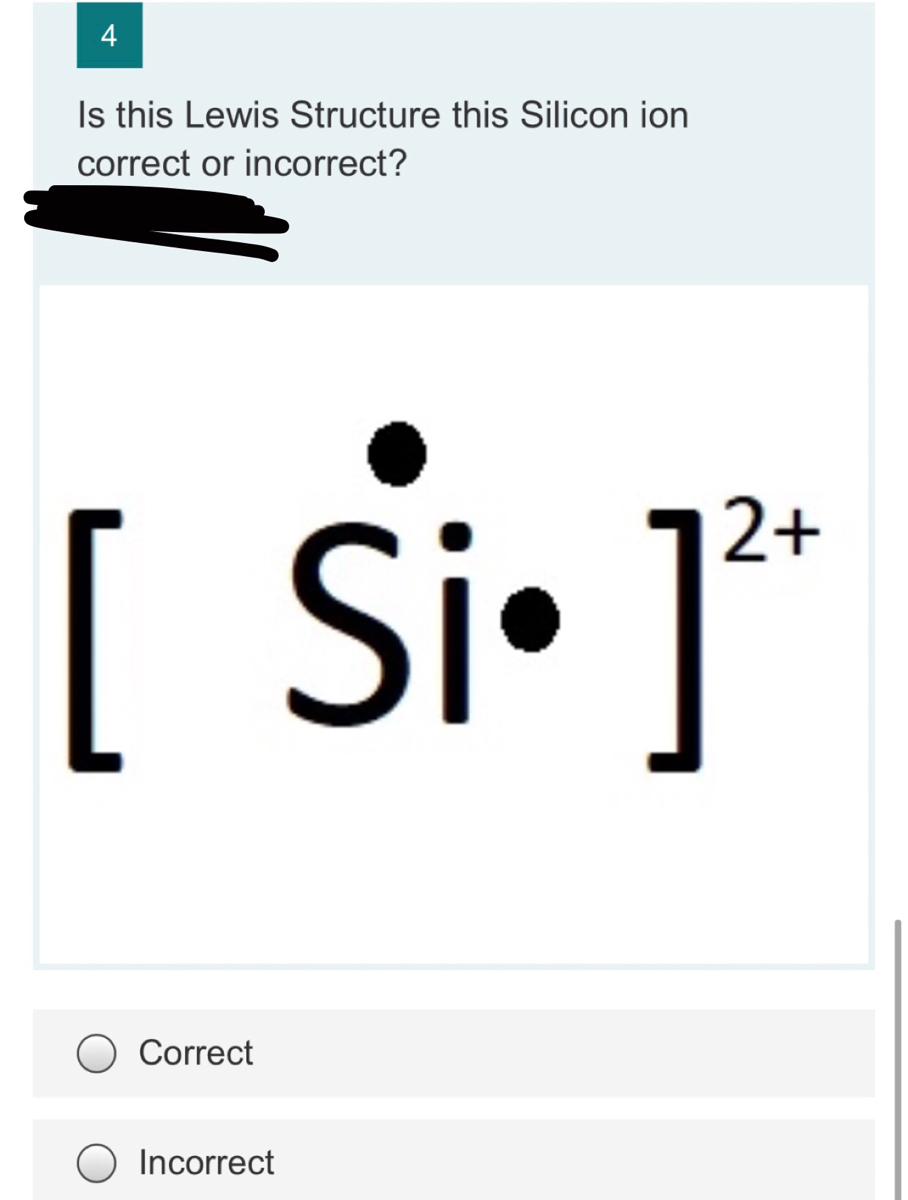




0 Response to "37 lewis dot diagram for silicon"
Post a Comment