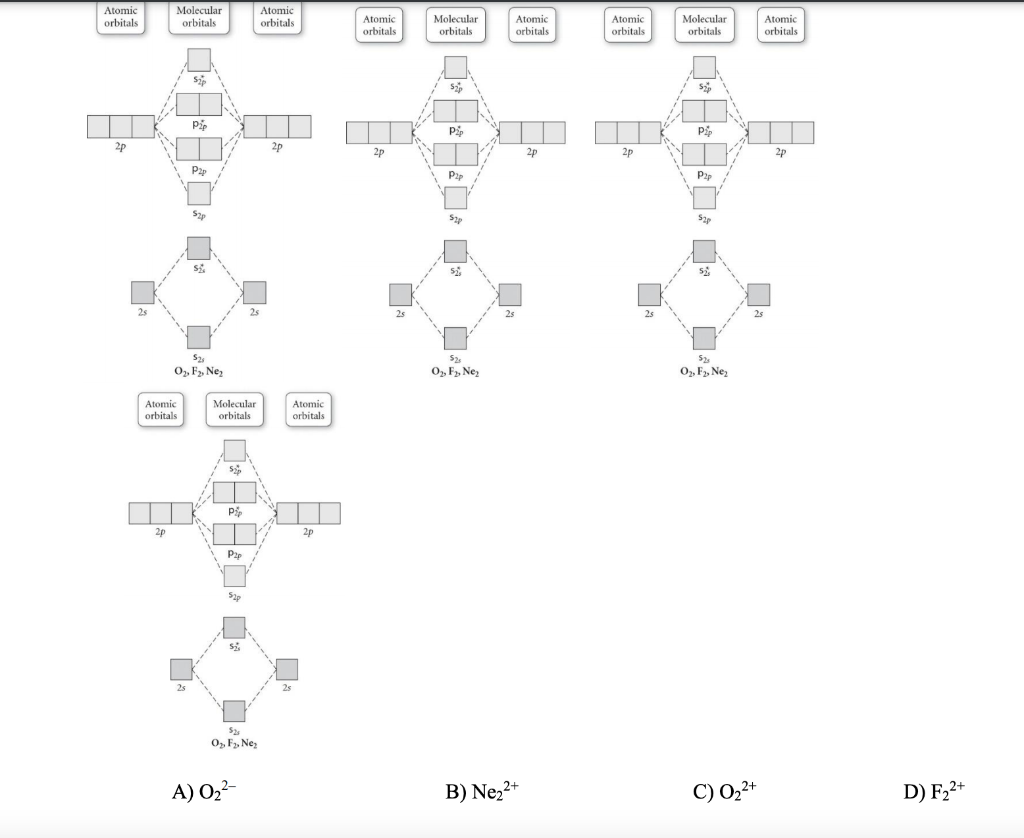
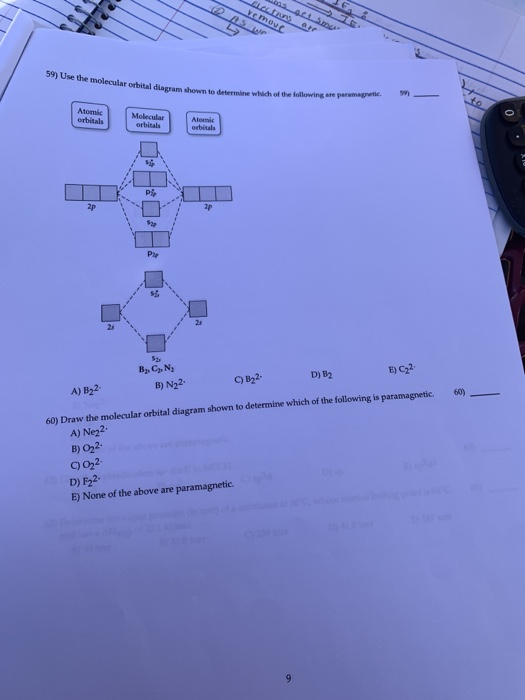
38 use the molecular orbital diagram shown to determine which of the following are paramagnetic.
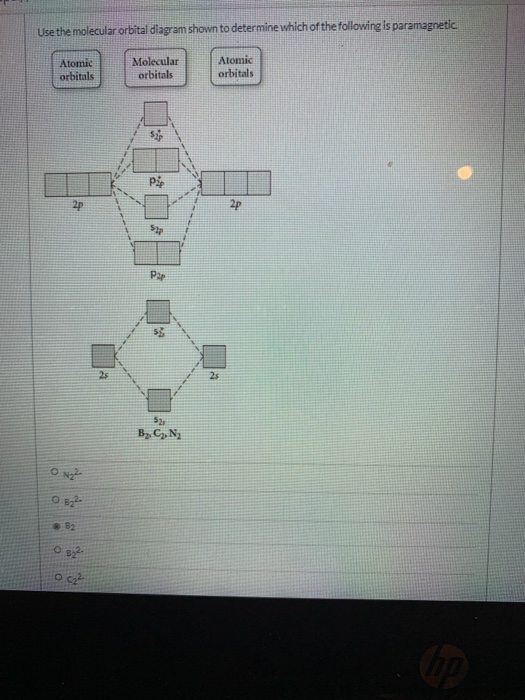
Transcribed image text: Use the molecular orbital diagram shown to determine which of the following is paramagnetic. (a) O^2-_2 (b) Ne^2+_2 (c) O^2-_2 (d) ... The energy needed to pair up two electrons in a single orbital is called the pairing energy (P). Electrons will always singly occupy each orbital in a degenerate set before pairing. P is similar in magnitude to Δ oct. When electrons fill the d orbitals, the relative magnitudes of Δ oct and P determine …
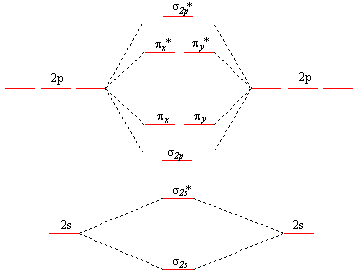
A fundamental factor in these molecular structures is the existence of electron pairs. These are electrons with opposed spins, allowing them to occupy the same molecular orbital without violating the Pauli exclusion principle (much like in atoms). Different molecular orbitals have different spatial distribution of the electron density.

Use the molecular orbital diagram shown to determine which of the following are paramagnetic.
1 answera. Only B2 B 2 is paramagnetic. All the others are diamagnetic. Their MO diagrams are shown below. b. For CO, the... Light-driven molecular oxygen reduction to H 2 O 2 from water is an emerging environmentally friendly approach that can convert solar energy into green chemical. In this work, the photocatalytic properties of g-C 3 N 4 for H 2 O 2 production was enhanced by co-modification with cyano group and SnO 2 nanocrystal through a facile one-step thermal polymerization method. 4 Jan 2021 — Use the molecular orbital diagram shown to determine which of the following are paramagnetic Atomic orbitals Molecular orbitals Atomic ...
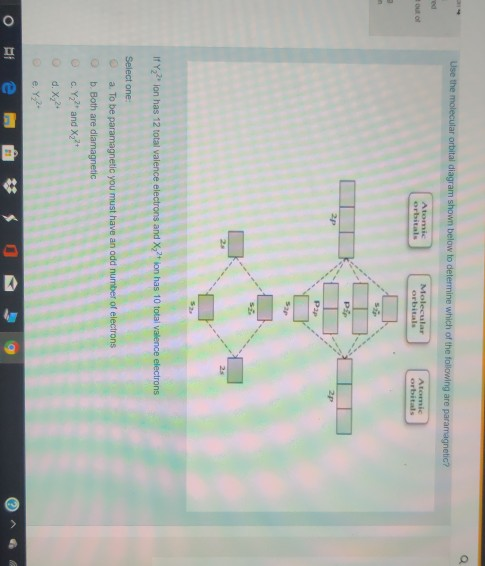
Use the molecular orbital diagram shown to determine which of the following are paramagnetic.. Fill in the molecular orbitals in the molecular orbital diagram for CO. One 2 s and three 2 p orbitals from carbon and one 2 s and three 2 p orbitals combine to form eight molecular orbitals in C O. The molecular orbitals in order of increasing energy are one sigma 2 s, one sigma 2 s star, two pi 2 p, one sigma 2 p, two pi 2 p star, and one ... Use the molecular orbital diagram shown to determine which of the following are paramagnetic: Atomic orbitals Molecular orbitals Atomic orbitals Pip OoFrNe}.4 answers · Top answer: So here we're just looking at a couple different orbital type. So looking on our s and R P Orbital's ... FREE Answer to Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. N22+ B22+...1 answer · Top answer: Electronic configuration of nitrogen is as follows N (7): 1s'2s 2p Nitrogen has five valence electrons, two electrons in 2s orbital and remaining 3 are ... 4 Oct 2018 · 1 answerProblem: Use the molecular orbital diagram shown to determine which of the following are paramagnetic.A. Ne22+ B. O22+ C. F22+ D. O22- E.
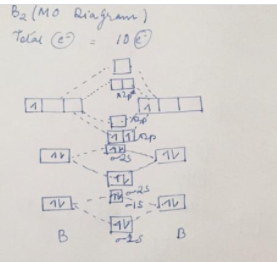
Use the molecular orbital diagram shown to determine which of following is paramagnetic. A) C2^2- B) B2 C) B2^2- D) N2^2+. E) B2^2+. Answer (1 of 4): It’s just the naming scheme for the hydrogen atom l = 2 wavefunctions in real form. When you solve the Schrodinger equation you do it in spherical coordinates and the solution you get out involve complex numbers. The levels 1s and 2s are shown as spheroids, while the three 2p orbitals are shown as split spheroids. Each full orbital has 2 electrons, yielding 10 total for this element. In the periodic table, there are 2 electrons in period 1, while both periods 2 and 3 have 8 electrons in the filled level. Academia.edu is a platform for academics to share research papers.
2 Oct 2021 — Use the molecular orbital diagram shown to determine which of the following is most stable. a. f22+ b. ne22+ c. f22- d. o22+ e. f2. Academia.edu is a platform for academics to share research papers. 4 Jan 2021 — Use the molecular orbital diagram shown to determine which of the following are paramagnetic Atomic orbitals Molecular orbitals Atomic ... Light-driven molecular oxygen reduction to H 2 O 2 from water is an emerging environmentally friendly approach that can convert solar energy into green chemical. In this work, the photocatalytic properties of g-C 3 N 4 for H 2 O 2 production was enhanced by co-modification with cyano group and SnO 2 nanocrystal through a facile one-step thermal polymerization method.
1 answera. Only B2 B 2 is paramagnetic. All the others are diamagnetic. Their MO diagrams are shown below. b. For CO, the...
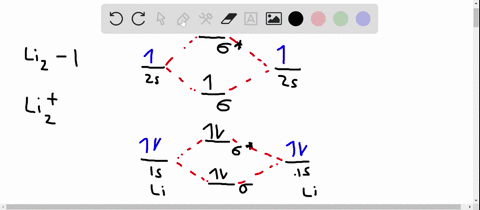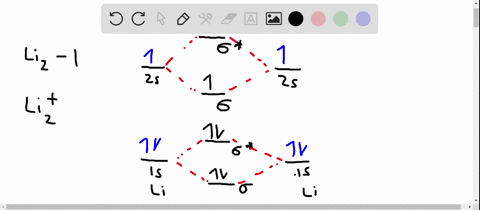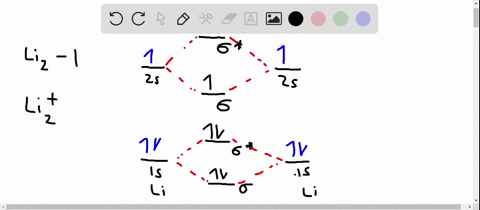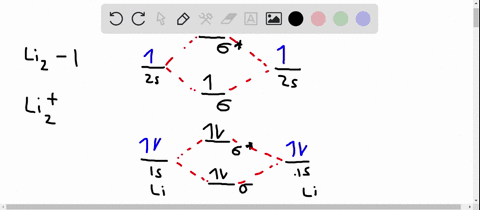
Solved Use The Molecular Orbital Diagram To Determine Which Of The Following Is Are Paramagnetic Select All That Apply Clz Siz Sb2 None Of These Is Paramagnetic Submit Request Answer
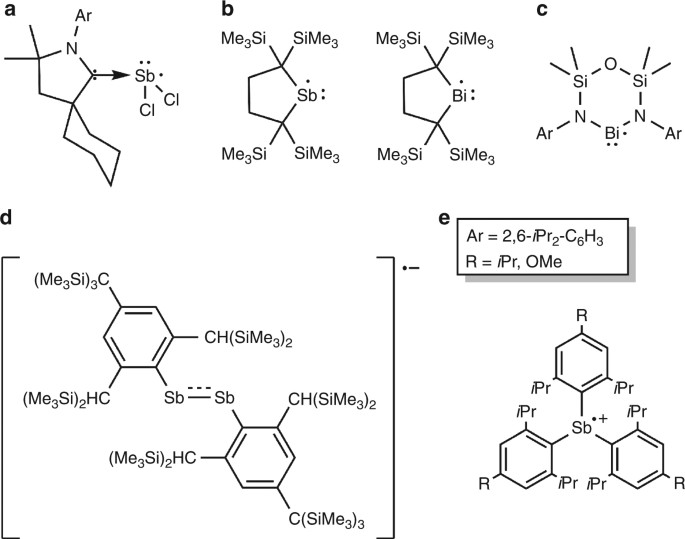
From Stable Sb And Bi Centered Radicals To A Compound With A Ga Sb Double Bond Nature Communications

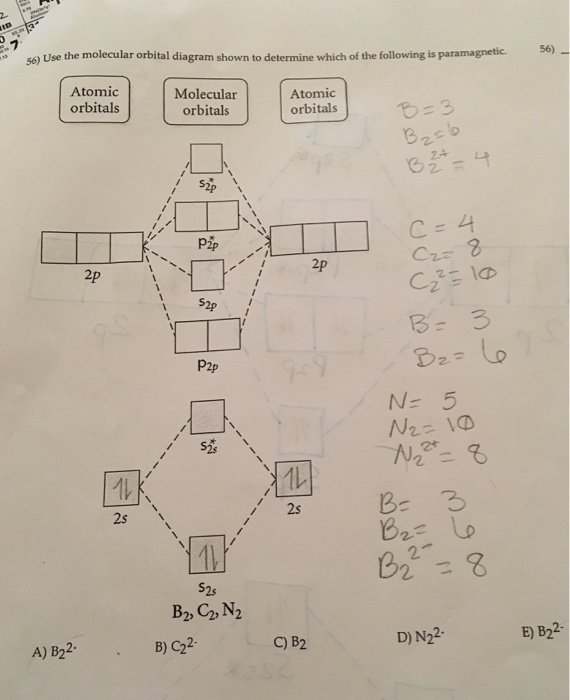
Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic N22 B22 Homeworklib

Why Is Molecular Oxygen Paramagnetic Inspite Of Having Even Number Of Electrons Discuss The Reason In Details Chemistry 7787105 Meritnation Com

Consider The Molecular Orbital Diagram For The Ion O 2 2 Predict The Bond Order A 3 0 B 2 5 C 1 0 D 2 0 E 1 5 Consider The Following Statements Will The Ion Be Paramagnetic Or Study Com

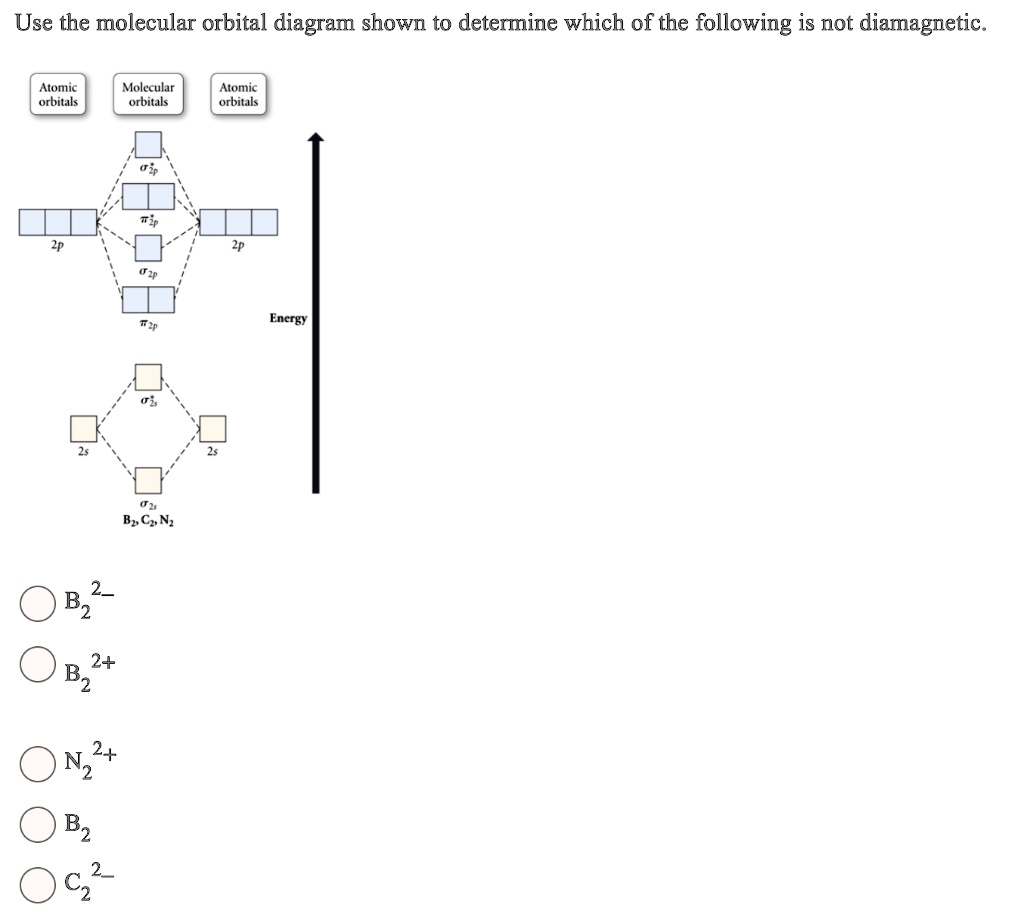
Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic Study Com
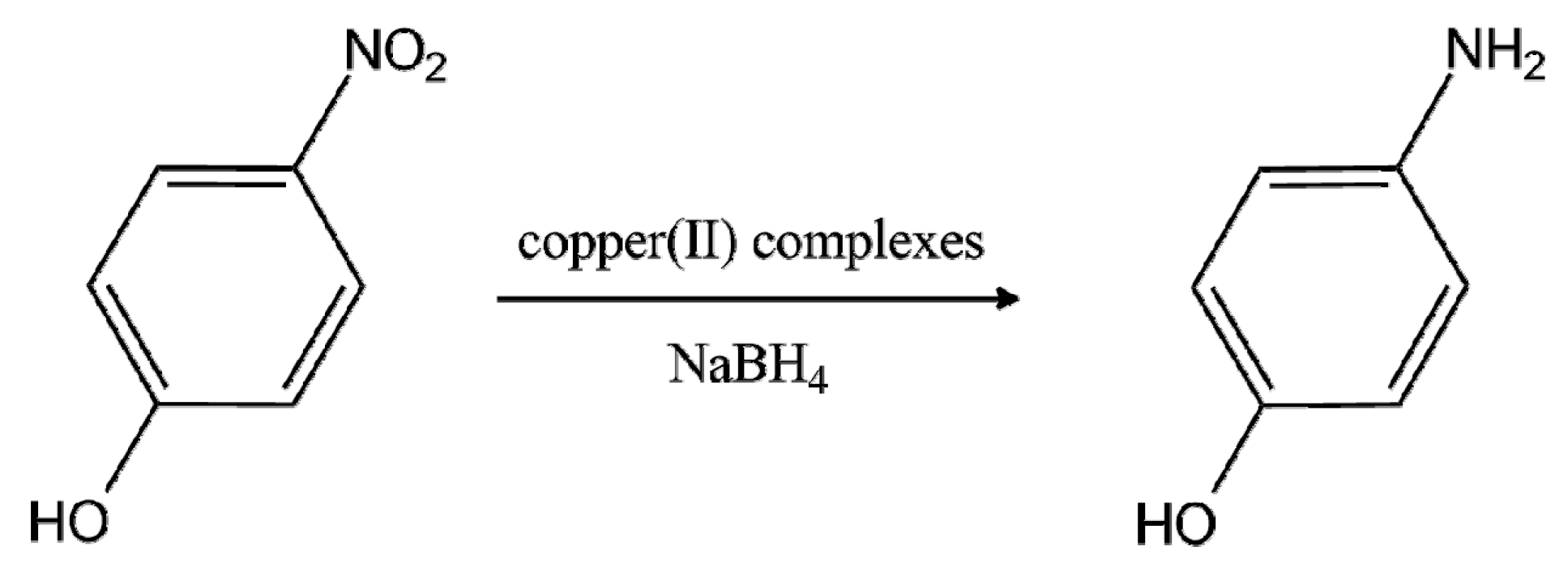
Molecules Free Full Text Efficient Catalytic Reduction Of 4 Nitrophenol Using Copper Ii Complexes With N O Chelating Schiff Base Ligands Html
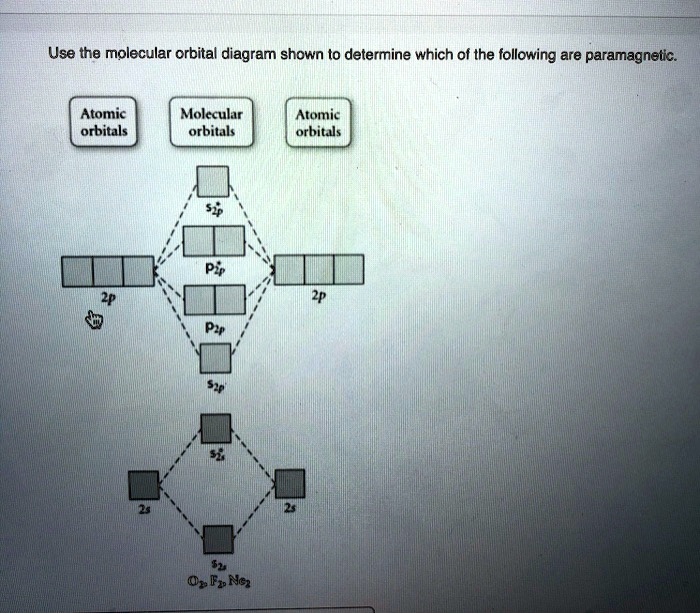
Solved Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Are Paramagnetic Atomic Orbitals Molecular Orbitals Atomic Orbitals Pip Oofrne

Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Most Stable A Homeworklib
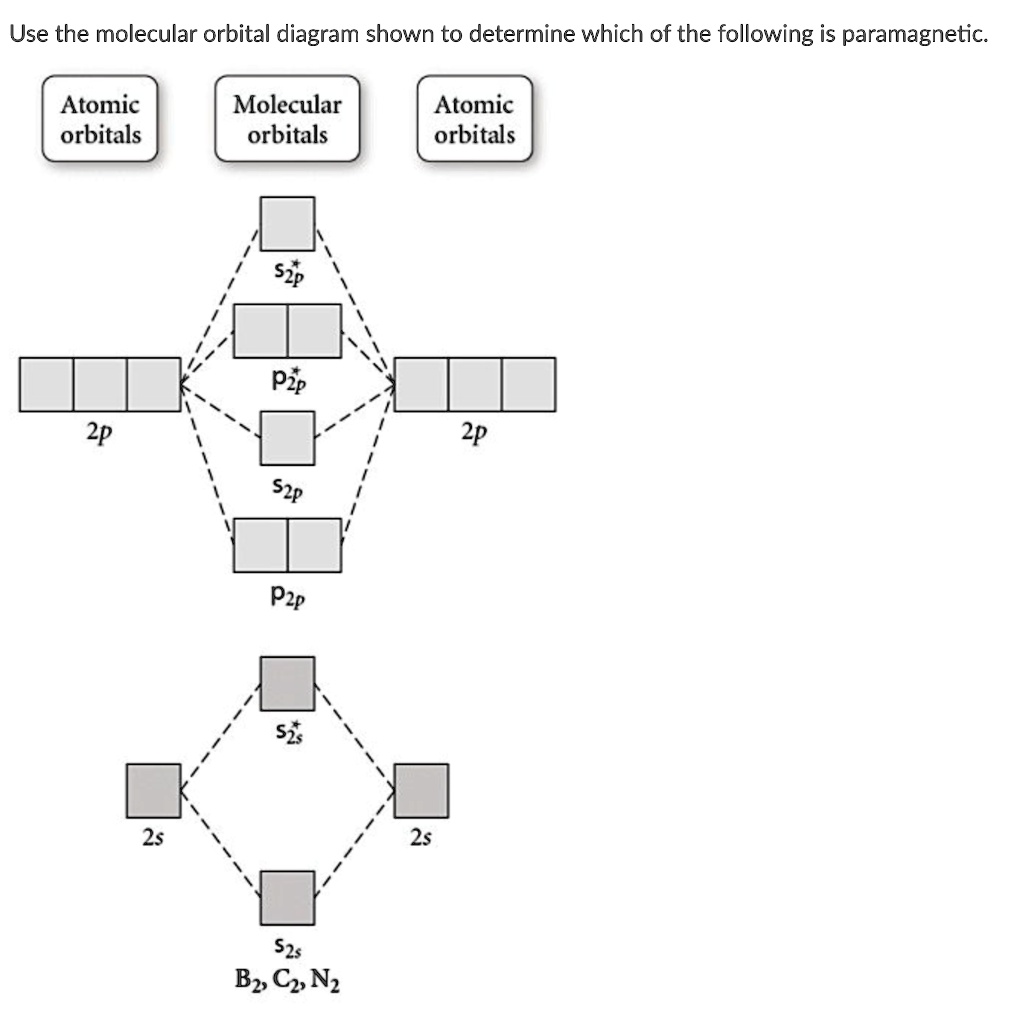
Solved Use The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic Atomic Orbitals Molecular Orbitals Atomic Orbitals S2p Pip 2p 2p S2p Pzp 2s 2s 52s Bz Cznz

A Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic B 2 2 B2 C 2 2 B 2 2 And N 2 2 B Draw The Lewis Structures And Molecular Orbital Diagrams For















0 Response to "38 use the molecular orbital diagram shown to determine which of the following are paramagnetic."
Post a Comment