41 orbital diagram for titanium
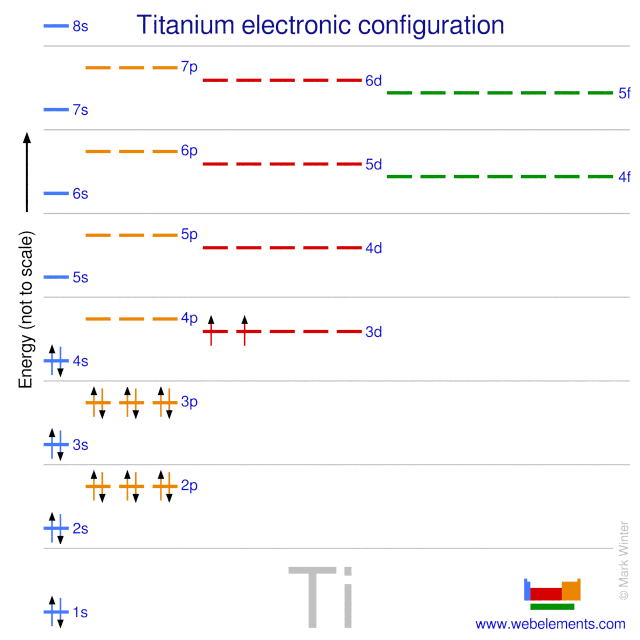
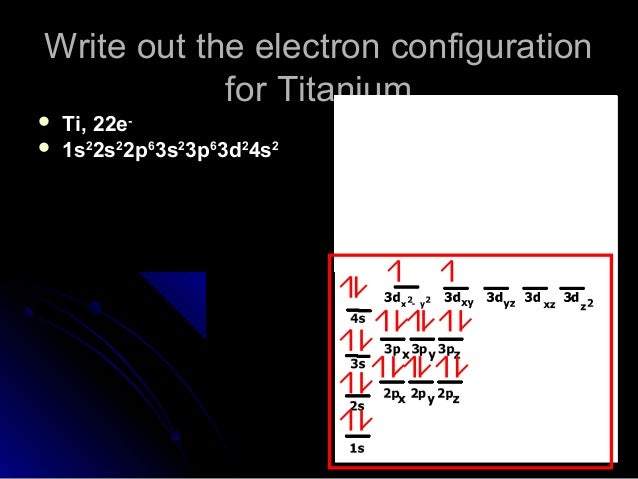
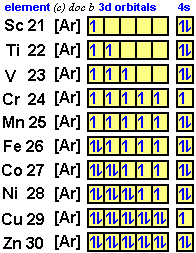
The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells. These energy levels contain sub-shells, or orbitals, each of ... on Orbital Diagram For Ti2+. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? The probability of finding an electron at the nucleus is 0. However, once the 4s orbital is filled, it becomes higher in energy than the 3d orbitals. This means that when titanium loses electrons, it does so.
Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. The eighth element in the periodic table is oxygen.

Orbital diagram for titanium
January 21, 2019 - Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams. Here are today’s CHM 110 notes for section 1 (MM 9:30 AM lecture). These notes discuss limiting reactant, percent yield, and basic thermodynamics and calorimetry · Any questions? Any trouble accessing the notes? Ask questions or report problems using the “Leave a reply” link Electronic configuration of the Titanium atom. Valence electrons. Orbital diagram
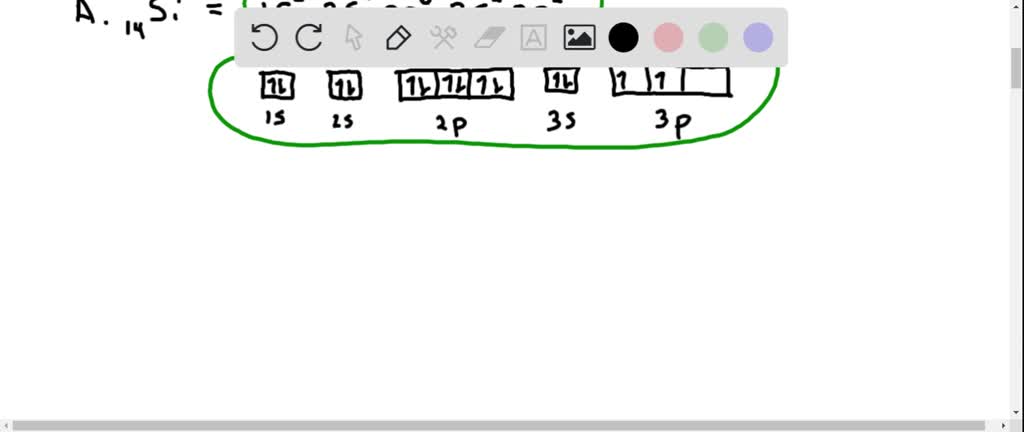
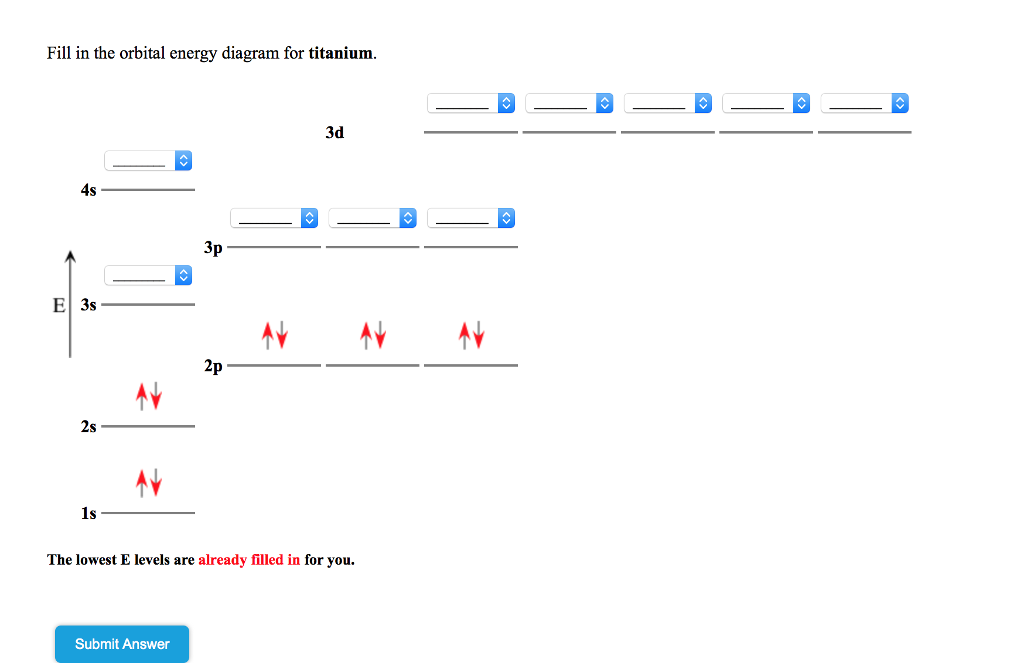
Orbital diagram for titanium. Answer to: Fill in the orbital energy diagram for titanium. The lowest E levels are already filled in for you. This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble... To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number... Draw the orbital diagram for the following elements: Oxygen (O) Titanium (Ti) Silicon (Si) Copper (Cu) For each of the following elements, identify if the electron configuration is correct or incorrect. If it is incorrect, give the fix to the configuration. Carbon (C) = 1s22s22p2. Sulfur (S) = 1s22s22p63p6
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation Titanium Electronic configuration. Electronic configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 2 >> Back to key information about the elementBack to key information about the element Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium ... Answer to Construct the orbital diagram of each atom or ion. What is the electron configuration for titanium? What is the electron...
orbital #4s, so you can see here However, once the #4s # orbital is filled, it becomes higher in energy than the orbital#3d #. This means that when titanium loses electrons, it does so from #4s # orbital first. #Ti: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^2 4s^2# Therefore, the two electrons that get lost when Let's consider titanium (Z = 22). Its electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2, which the (n + l) rule correctly predicts. If the electron configuration depended solely on the orbital energies, we would expect: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 4 - with no electrons in the 4s orbital. In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation Titanium has 1s, 2s, 2p, 3s ,3p, 3d, 4s orbitals in which electrons are present. The valence electrons present in valence shells, 3d and 4s are 4. These ...1 answer · Top answer: Titanium is a transition element which has atomic number 22. It forms various types of compounds like titanium tetrachloride and trichoride etc. It is popularly ...

Year Group Poster Review For Chemistry Task 1 In The Center Of The Poster Draw A Periodic Table Outline And Label It With 7 Different Types Of Metals Ppt Download
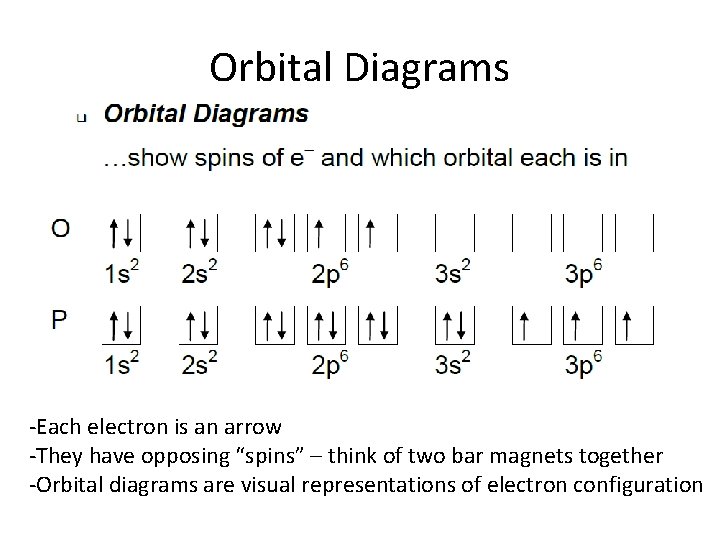
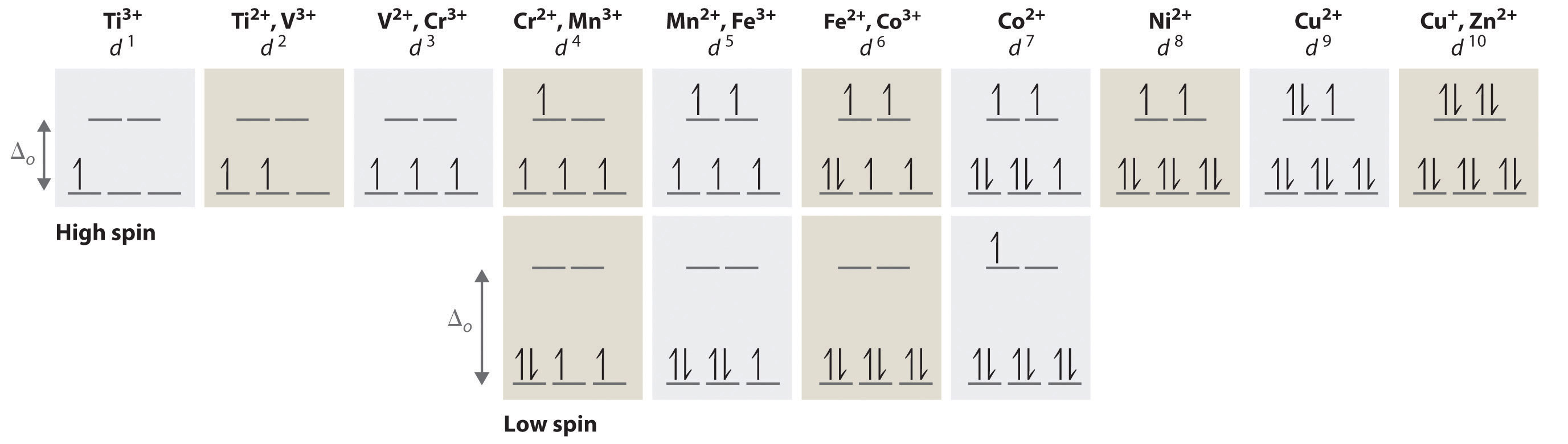
What are the rules for filling out orbital diagrams? Hund's rule states that the most stable configuration is the one with the highest possible number of parallel spins. This means that when writing orbital diagrams for partially full shells, fill in all of the up-spin electrons before adding any down-spin electrons.

Solved Write The Electron Configuration Full Or Condensed For Titanium And Selenium And Based On The Electron Orbital Diagram Indicate How Many Unpaired Valence Electrons There Are In The Elements And In What
Titanium is a metal. It's not just any metal, it's a transition metal. Being a transition metal, it has a special electron configuration. It adds its next electron to the third shell, not the outermost fourth shell. With a configuration of 2-8-10-2, titanium is out in the world and ready to bond with other elements.
Choose the orbital diagram that represents the ground state of N. orbital diagram where 1s and 2s orbitals contain 1 pair of electrons each. 2p orbitals are empty. orbital diagram where 1 s and 2 s orbitals contain 1 pair of electrons each. 2 p orbitals contain 3 pairs of electrons.
Answer to Construct the orbital diagram of each atom or ion.TiTi2+Ti4+...
What is the correct orbital diagram for phosphorus? The p orbital can hold up to six electrons. We’ll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we’ll move to the 3p where we’ll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s22s22p63s23p3.
Titanium Electron Configuration (Ti) with Orbital Diagram. Titanium Electron Configuration: Titanium is a chemical element that has a chemical symbol Ti. Its atomic number is 22. It is a transition metal lustrous which has a silver colour, low density, and high strength. It is resistant to corrosion in aqua regia, sea water, and chlorine.
A good place to start when trying to figure out the electron configuration of an ion is the electron configuration of the neutral parent atom.. In this case, titanium, #"Ti"#, is located in period 4, group 4 of the periodic table and has an atomic number of #22#. This means that a neutral titanium atom will contain #22# protons in its nucleus and #22# electrons surrounding its nucleus.
Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the data : ... Accept appropriate diagram for M1, M2 or both. Do not give marks for answers that refer to the lines in the spectrum. ... Sketch the orbital diagram of the valence shell of a bromine atom (ground state) on the energy axis provided.
Elements zinc and germanium Atomic Basic Information a Lewis dot structure for metal... Orbital diagram of titanium and the would seldom write a Lewis dot structure for titanium metal Format Citing. Gallium is located in the fourth period ( row ) of the atom complex...
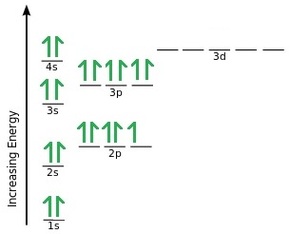
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
Answer: Go to the following website to find the orbital diagram of any element of any Oxidation state. Orbital Energy Diagram and Atomic Electron Configuration Tool As for an actual diagram (per Wiki) is above. You can also find the electron configuration on both websites. For an overview and ...
Electron binding energies for titanium. All values of electron binding energies are given in eV. The binding energies are quoted relative to the vacuum level for rare gases and H 2, N 2, O 2, F 2, and Cl 2 molecules; relative to the Fermi level for metals; and relative to the top of the valence band for semiconductors. Label Orbital eV ...
Titanium is the ninth most abundant element on Earth. It is almost always present in igneous rocks and the sediments derived from them. It occurs in the minerals ilmenite, rutile and sphene and is present in titanates and many iron ores. Titanium is produced commercially by reducing titanium (IV) chloride with magnesium.
Orbital Diagram. 1s ... Titanium dioxide (TiO2), a white pigment that covers surfaces very well, is used in paint, rubber, paper and many others. Sources Usually occurs in the minerals ilmenite (FeTiO3) or rutile (TiO2). Also in Titaniferous magnetite, titanite (CaTiSiO5), and iron ores. Pure metal produced by heating TiO2 with C and Cl2 to ...
Exam 4 Review: Ch.8-9. Electromagnetic radiation with a wavelength of 745 nm appears as red light to the human eye. The energy of one photon of this light is ________ J. Calculate the wavelength (in nm) of the blue light emitted by a mercury lamp with a frequency of 6.19 × 10^14 Hz. Nice work!
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
Orbital Diagram For Chromium Electron Configurations In The 3d Orbitals. Orbital Diagram For Chromium Exam 2013 Chem1010 Fundamentals Of Chemistry Studocu. Orbital Diagram For Chromium Give Electronic Configuration And Orbital Diagram Of Titanium And
For that, we have electron shell diagrams. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. For each electron shell atom diagram, the element symbol is listed in the nucleus. This is a Bohr Diagram of a titanium atom. The nucleus of a titanium atom has 22 protons and 26 neutrons.
0 Comments. on Orbital Diagram For Germanium. orbital. Because an electron can have either one of two spins, any orbital can hold a maximum of four . The orbital diagram for germanium is. 1s. 2s. 2p. 3s. Oxidation States, +4,2. Electrons Per Shell, 2 8 18 4. Electron Configuration, [Ar] 3d10 4s2 4p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2.
2 weeks ago - The purpose of introducing quantum numbers has been to show that similarities in the electron arrangement or electron configuration lead to the similarities and differences in the properties of …
November 25, 2020 - Ti Titanium Element information, facts. Titanium properties, uses and trends | Periodic Table of the Elements - complete information about the titanium element - Facts, atomic mass, melting point, How to Locate on Periodic Table, History, Abundance, Physical Properties, Thermal Properties, ...
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
Solved According To Hund S Rule And The Aufbau Principle The Electron Configuration For The Titanium Atom Can Best Be Expressed As
Orbital notation shows the number of electronics in an orbit. The orbital notation of Hydrogen is a circle with one slash through it. The electron configuration of Hydrogen is 1(s^1).
Electronic configuration of the Titanium atom. Valence electrons. Orbital diagram
Here are today’s CHM 110 notes for section 1 (MM 9:30 AM lecture). These notes discuss limiting reactant, percent yield, and basic thermodynamics and calorimetry · Any questions? Any trouble accessing the notes? Ask questions or report problems using the “Leave a reply” link
January 21, 2019 - Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.

Orbital Diagrams Construct The Orbital Diagram Of Each Atom Or Ion What Is The Electron Configuration Homeworklib

Ti Titanium Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids
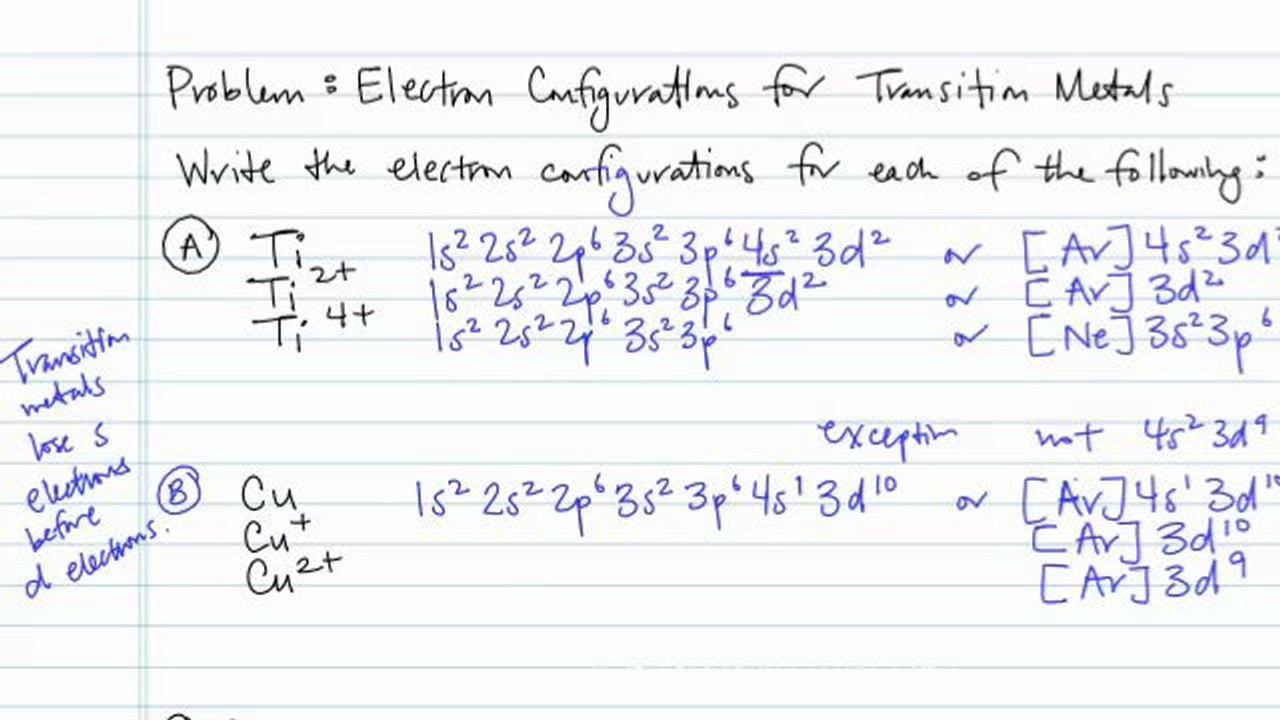
Electron Configurations For Transition Metals And Their Ions Problem Concept Chemistry Video By Brightstorm



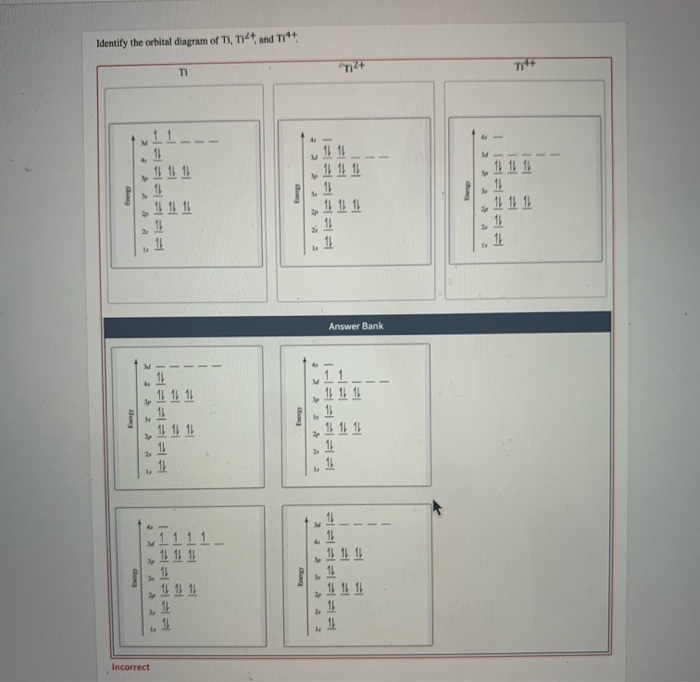








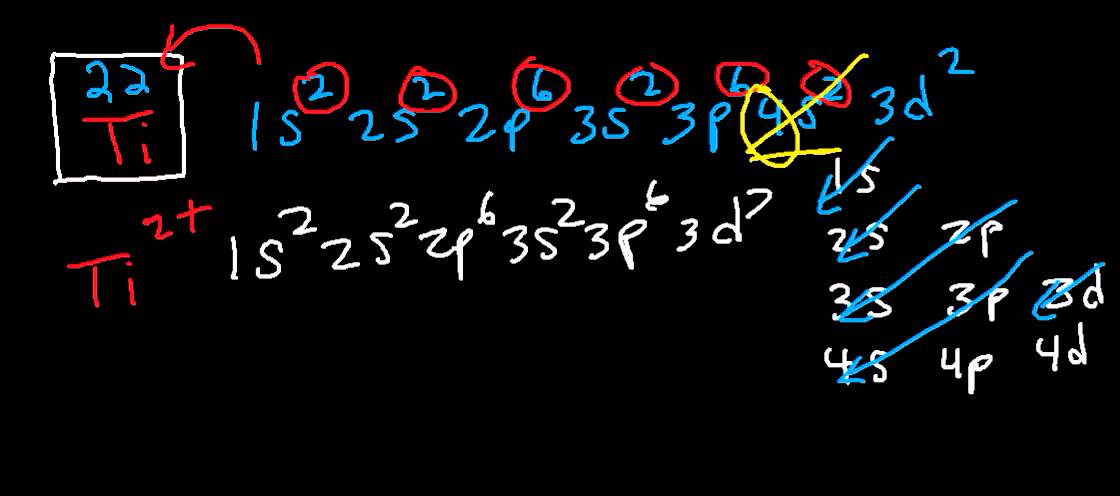











0 Response to "41 orbital diagram for titanium"
Post a Comment