42 construct the octahedral crystal-field splitting diagram
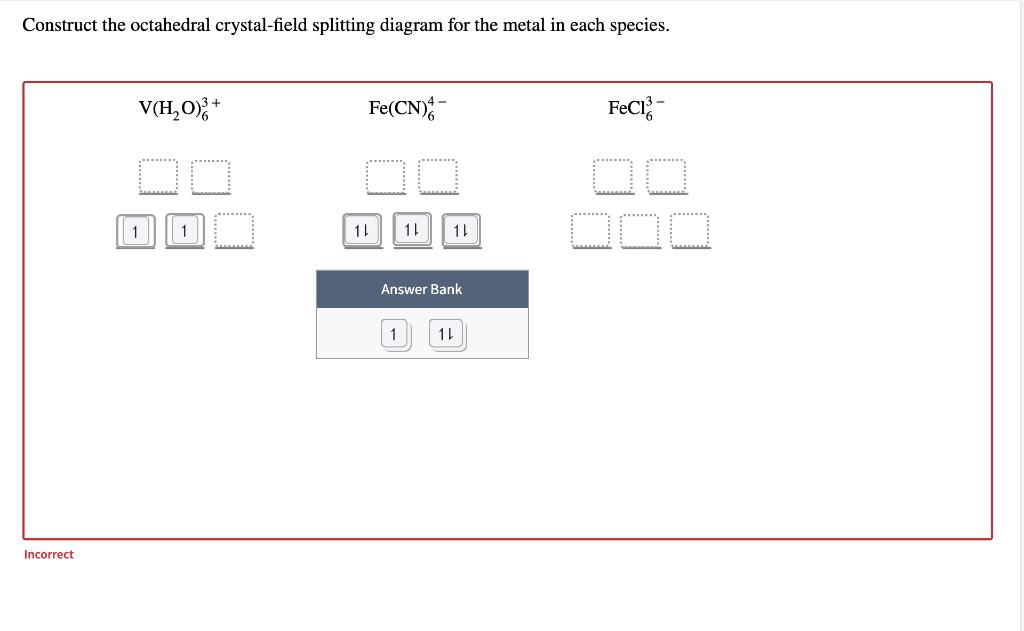
Construct the octahedral crystal-field splitting diagram for the metal in each species. You are currently in a labeling module. Turn off browse mode or quick nav, Tab to items, Space or Enter to pick up, Tab to move, Space or Enter to drop. V(H2O)3+6 Fe(CN)4−6 Mn(H2O)2+6 Construct the octahedral crystal field splitting diagram for the metal in each species. Construct the octahedral crystal field splitting diagram for the metal in each species. Just remember that color depends on which electronic transitions are available to the species in question.
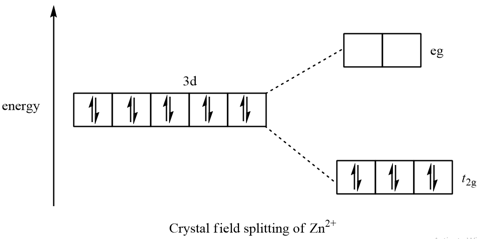
The octahedral crystal field splitting diagram for is as followscthe electron configuration of is. 1 draw the octahedral crystal field splitting diagram for each metal ion. Fe2 low spin is broken down into a number of easy to follow steps and 23 words.
Construct the octahedral crystal-field splitting diagram
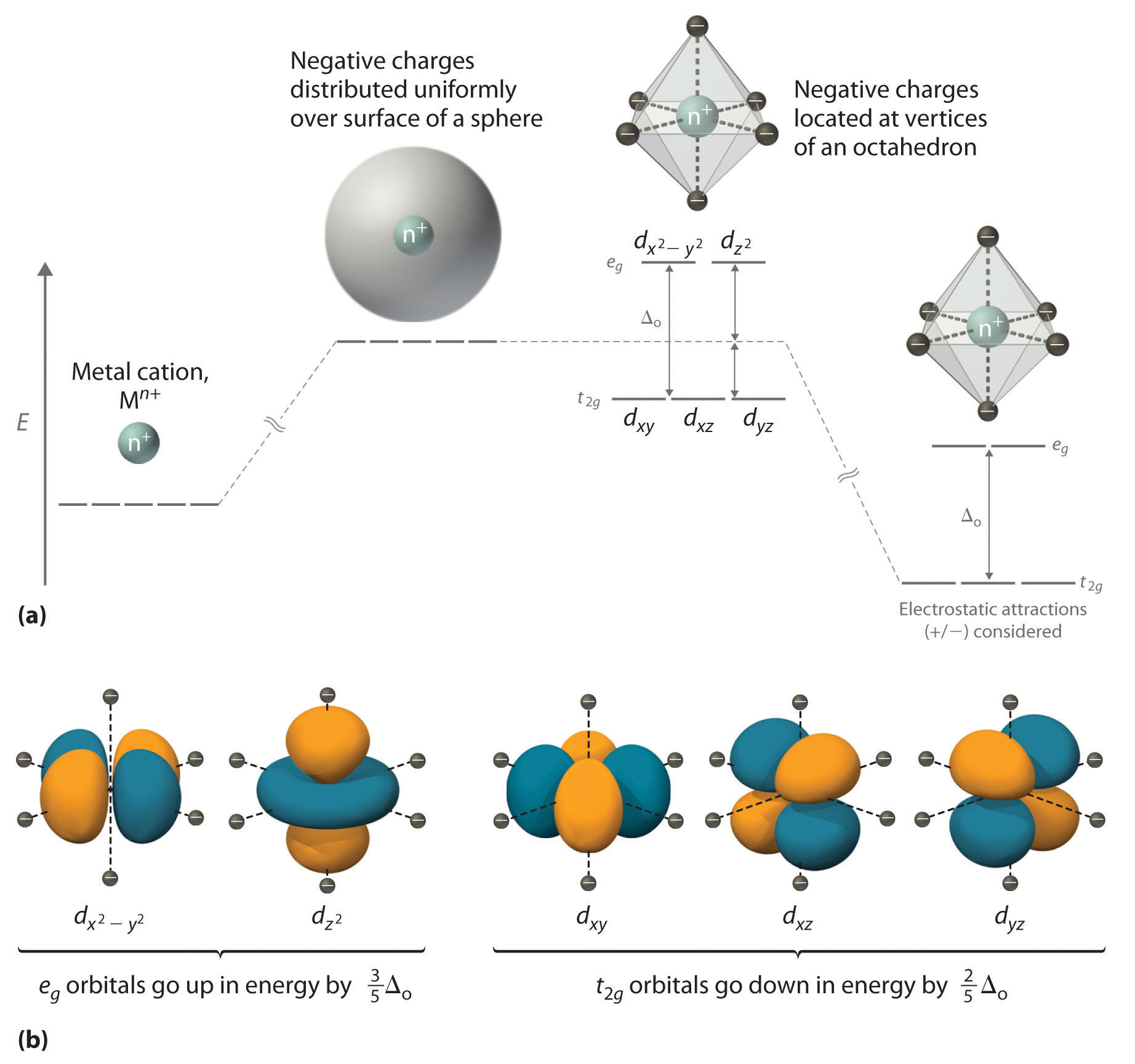
Solid State ChemiStry and itS appliCation 2014 Anthony R. West In octahedral symmetry the d-orbitals split into two sets with an energy difference, Δ oct (the crystal-field splitting parameter, also commonly denoted by 10Dq for ten times the "differential of quanta") where the d xy, d xz and d yz orbitals will be lower in energy than the d z 2 and d x 2-y 2, which will have higher energy, because the ... Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+. A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm. a) Calculate the crystal-field splitting energy, Δ, in.
Construct the octahedral crystal-field splitting diagram. Crystal field theory was established in 1929 treats the interaction of metal ion and ligand as a purely electrostatic phenomenon where the ligands are considered as point charges in the vicinity of the atomic orbitals of the central atom. Development and extension of crystal field theory taken into account the partly covalent nature of bonds ... Construct the octahedral crystal field splitting diagram for the metal in each species. Lecture 9 crystal field theory for octahedral. Crystal Field Theory Chemistry Libretexts Cfse the stability that results from placing a transition metal ion in the crystal field generated by a set of ligands. Construct the octahedral crystal field splitting diagram for the metal in each species. Since the oxalate ligand is fairly low in the series a weak field ligand at this point you may not have studied ligand field theory yet which explains why it is a weak ligand. Cr4 mnh2o62 asked by katie on march 30 2012 chemistry based on crystal field ... Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Stefanos Mourdikoudis ab, Roger M. Pallares ab and Nguyen T. K. Thanh * ab a Biophysics Group, Department of Physics and Astronomy, …
The shape/ structure of [XeF5]– and XeO3F2, respectively, are : [Main Sep. 02, 2020 (II)] (a) pentagonal planar and trigonal bipyramidal (b) octahedral and square pyramidal (c) trigonal bipyramidal and pentagonal planar (d) trigonal bipyramidal and trigonal bipyramidal 5. The molecular geometry of SF6 is octahedral. What is the geometry of SF4 (including lone pair(s) of electrons, if any ... Their blank d -splitting diagrams within the realm of crystal field theory are: [Ni(CN)4]2−: The d orbitals fill with 8 electrons, then, with a low spin configuration. You can see that an even number of d orbitals will get filled ( dyz,dxz,dz2,dxy) with an even number of 3d electrons. This gives rise to a diamagnetic configuration, as expected. FREE Expert Solution. Octahedral crystal-field splitting diagram → d-orbital electrons. high-spin - electrons can occupy the upper level (eg) low-spin - electrons can pair up with the electrons on the lower level (t2g) Recall that: weak field ligands → high spin → lowΔ or crystal field splitting energy values. Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ This board is unable to make drawings/diagrams available. species coefficient is "1" then "1" needs to be entered in the field before that species. Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species.
Construct the octahedral crystal field splitting diagram for the metal in each species. 3 for each species molecule or ion in the net ionic equation assign oxidation. A d1 octahedral complex is found to absorb visible light with the absorption maximum occcurring at 523 nm. Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ Mn (H2O)6^2+. Nov 14, · Basically, the question is referring to the compound K3 [Fe (C2O4)3]. It asks what is the electron configuration in this comound, I got it to be d5. Fe in the compound is Fe (III) so 23 electrons -> d5. Construct the octahedral crystal field splitting diagram for the metal in each species. Construct the octahedral crystal field splitting diagram for the metal in each species. H2o62 has 6 d electrons. Basically the question is referring to the compound k3fec2o43. Cr4 mnh2o62 20607 results page 5. Match the terms with the correct definitions. The Forum on Renaissance in NO Chemistry presents 14 contributions, of which a few focus on NO chemistry itself and the remaining papers focus on the interaction of metal complexes with NO. The broad scope of topics presented in this Forum illustrates NO's rich chemistry, in which inorganic and bioinorganic chemists are taking the field to new and exciting levels.
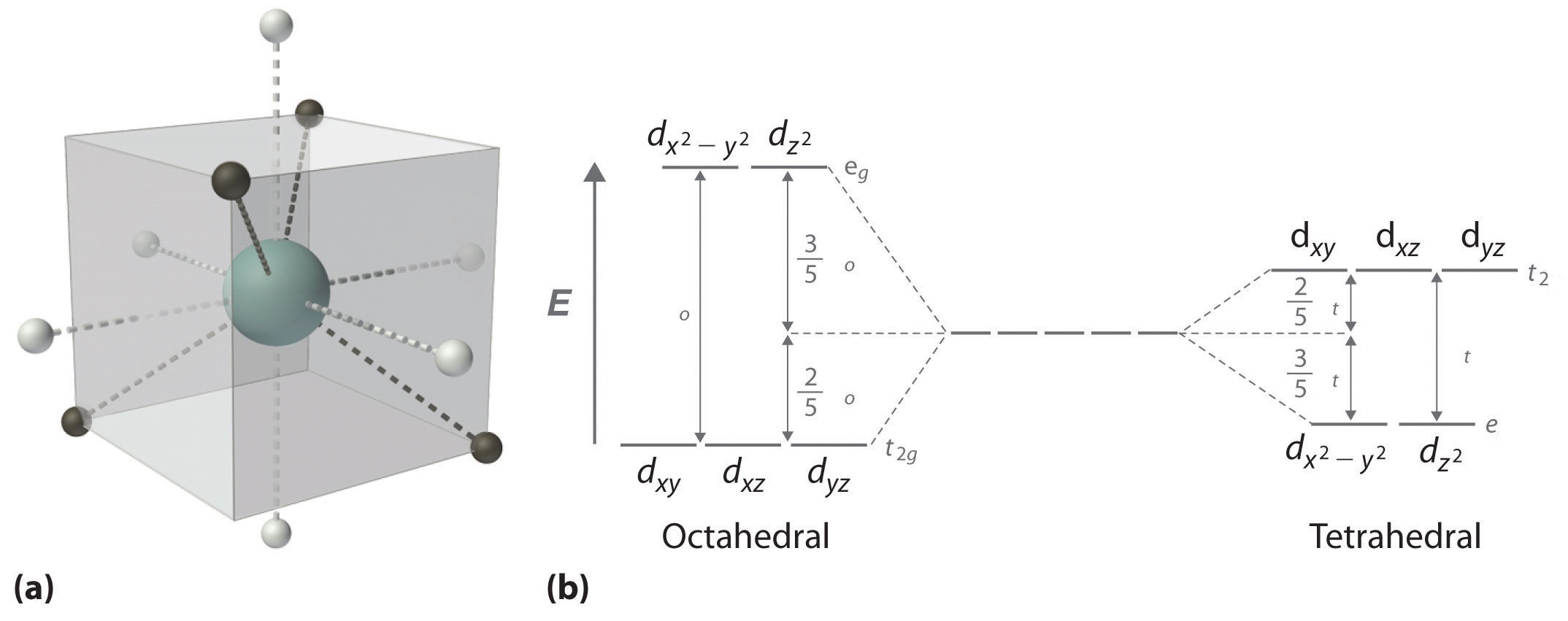
The difference between the energies of the t 2g and e g orbitals in an octahedral complex is represented by the symbol o.This splitting of the energy of the d orbitals is not trivial; o for the Ti(H 2 O) 6 3+ ion, for example, is 242 kJ/mol. . The magnitude of the splitting of the t 2g and e g orbitals changes from one octahedral complex to another. It depends on the identity of the metal ion ...
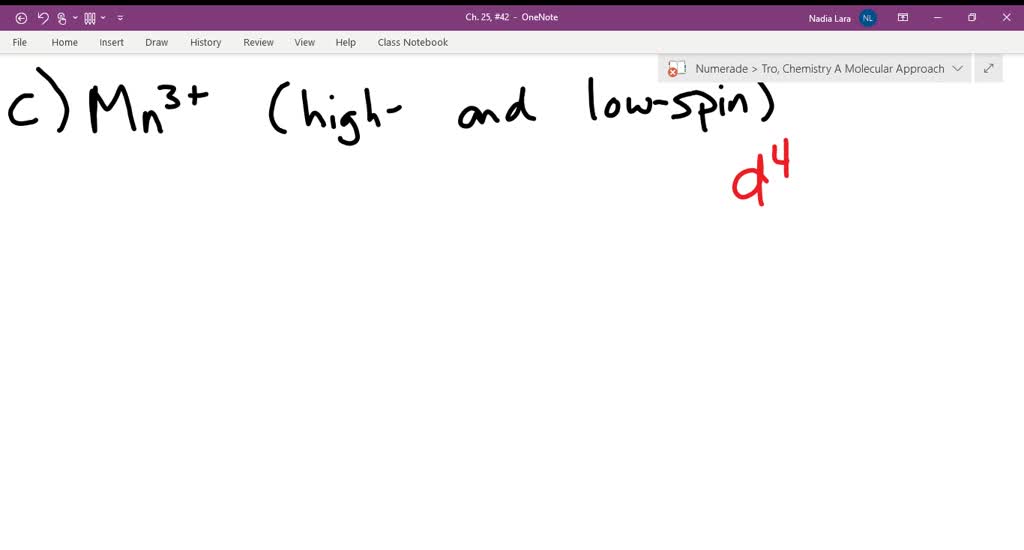
Solved Construct The Octahedral Crystal Field Splitting Diagram For The Metal In Each Species V H O Fe Cn G Mn L O Amwtr Bant
Construct the octahedral crystal field splitting diagram for the metal in each species. If the splitting of the d orbitals in an octahedral field is δoct the three t2g orbitals are stabilized relative to the barycenter by 25 δoct and the eg orbitals are destabilized by 35 δoct.
Construct the octahedral crystal-field splitting diagram for the metal in each species. V ( H 2 O ) 3 + 6 V(H2O)63+ Co ( CN ) 3 − 6 Co(CN)63− Mn ( H 2 O ) 2 + 6 Mn(H2O)62+ Construct the octahedral crystal-field splitting diagram for the metal in each species.
Construct the octahedral crystal-field splitting diagram for the metal in each species. Subject: Chemistry Price: 2.85 Bought 3. Share With. Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+. rated 5 stars. Purchased 3 times. Completion Status 100%.
Octahedral Complexes In octahedral complexes, the molecular orbitals created by the coordination of metal center can be seen as resulting from the donation of two electrons by each of six σ-donor ligands to the d-orbitals on the metal. The metal orbitals taking part in this type of bonding are nd, (n+1)p and (n+1)s. It should be noted down
For octahedral complexes, crystal field splitting is denoted by . We find that the square planar complexes have the greatest crystal field splitting ligand field (left diagram) and the tetrahedral field (right diagram).D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with ...
Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the ...
How to draw the crystal field splitting diagram 2. Draw the octahedral crystal field splitting diagram for each metal ion. This diagram shows the field splitting of a metal with ligands in an octahedral configuration. For transition metal complexes called crystal field theory. Home question construct the octahedral crystal field splitting ...
In an octahedral complex, the d orbitals of the central metal ion divide into two sets of different energies. The separation in energy is the crystal field splitting energy, Δ. (A) When Δ is large, it is energetically more favourable for electrons to occupy the lower set of orbitals.

Draw Figure To Show Splitting Of D Orbitals In An Octahedral Crystal Field From Chemistry Coordination Compounds Class 12 Cbse
Answer to: Construct the octahedral crystal-field splitting diagram for the metal in each species. (A) Cr^{4+}. (B) Mn(H_2O)_6^{2+}. By signing up,...
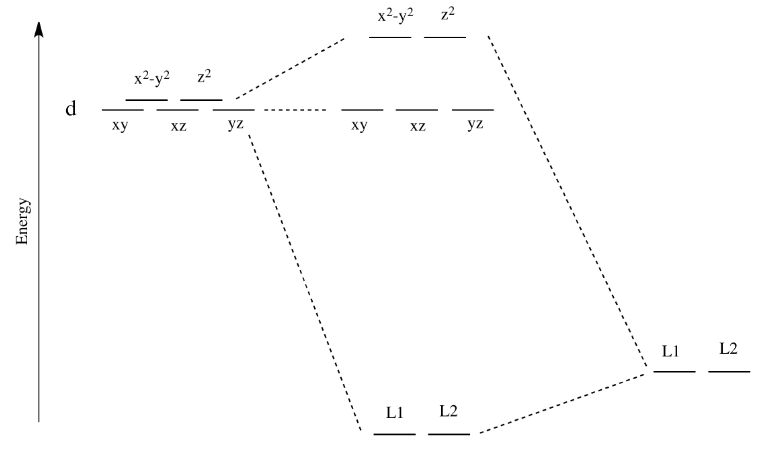
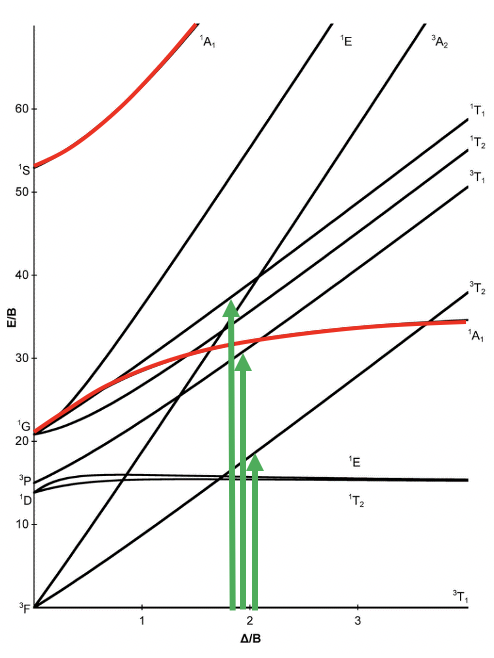
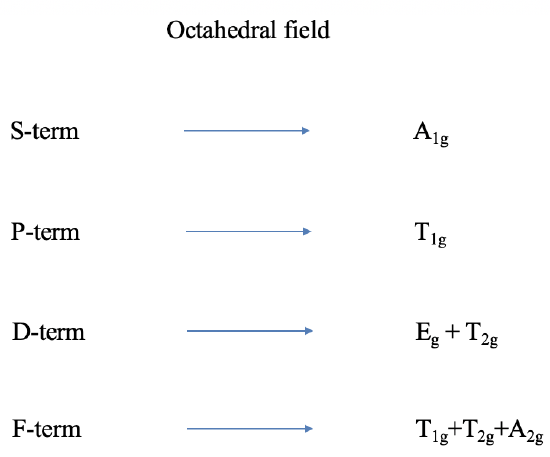
!To construct the correlation diagram, we look at two extremes: "Left side: A weak field , just strong enough to lift the R3 free-ion term degeneracies. • On the left side of the diagram we show the energies of the free-ion terms and the Mulliken symbols for the terms into which they are split in a weak octahedral field.
Academia.edu is a platform for academics to share research papers.
Solved Construct The Octahedral Crystal Field Splitting Diagram For The Metal In Each Species Course Hero
Its fundamental process is the creation of a Schrödinger wavefunction for an electron bound to a molecule: spatial distribution of the square of that wavefunction is then this theory's namesake construct. For ten points, name the bonding model whose namesake diagrams allow elucidation of the spin-pairing of electrons bound in a molecule and the existence of bonding and anti-bonding orbitals.
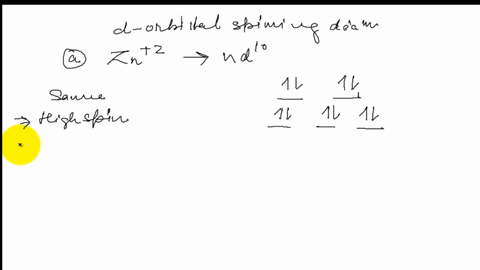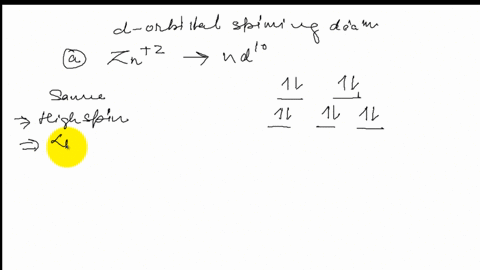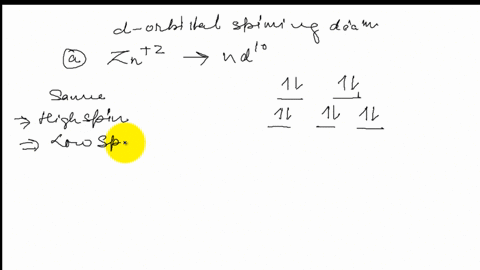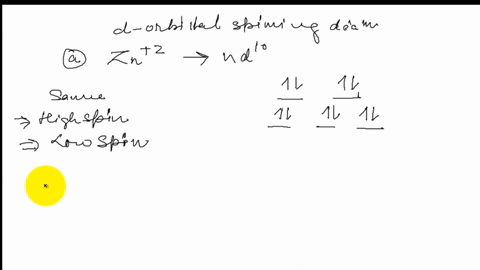
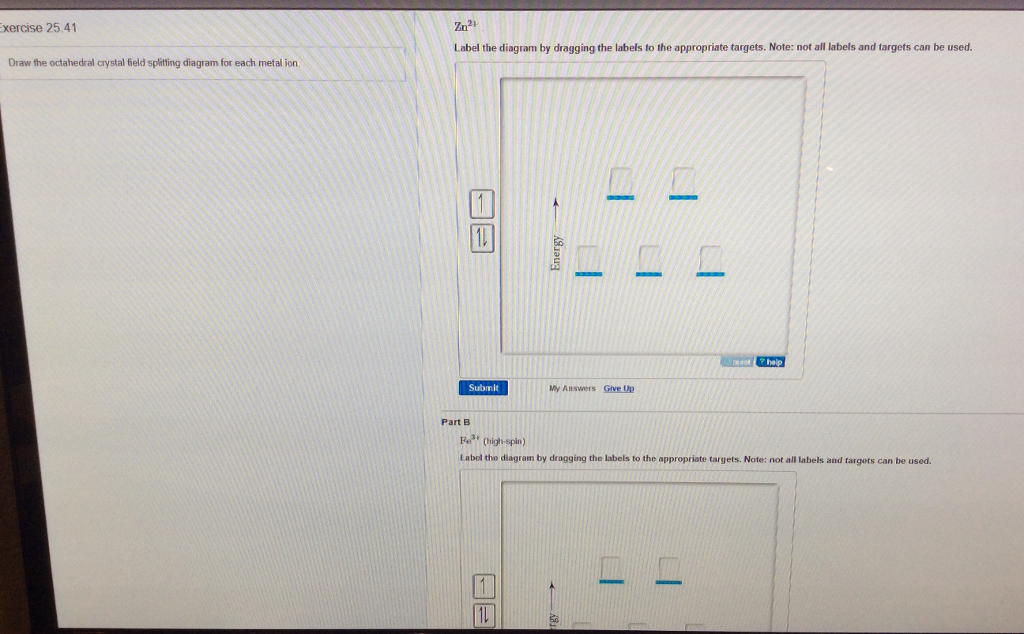
Solved Draw The Octahedral Crystal Field Splitting Diagram For Each Metal Ion A Zn2 B Fe3 High And Low Spin C V3 D Co2 High Spin
Construct the octahedral crystal field splitting diagram for the metal in each species. If the splitting of the d orbitals in an octahedral field is δoct the three t2g orbitals are stabilized relative to the barycenter by 25 δoct and the eg orbitals are destabilized by 35 δoct.
This video discusses the repulsion between the d-orbitals on the metal cation (orbitals meaning negative electrons "smeared out") and the electrons on the li...
Construct the octahedral crystal field splitting diagram. Called crystal field theory. If the splitting of the d orbitals in an octahedral field is δoct the three t2g orbitals are stabilized relative to the barycenter by 25 δoct and the eg orbitals are destabilized by 35 δoct. Basically the question is referring to the compound k3fec2o43.
D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral 2. Tetrahedral 3. Trigonal bipyramidal 4. Square pyramidal d z2x2-y d xy d yzxz 5. Square planar d z2x2-y d xy d yzxz d z2 d x2-yxy d yz d xz d z2 d x2-y2 d xy d yz d ...
Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+. A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm. a) Calculate the crystal-field splitting energy, Δ, in.
In octahedral symmetry the d-orbitals split into two sets with an energy difference, Δ oct (the crystal-field splitting parameter, also commonly denoted by 10Dq for ten times the "differential of quanta") where the d xy, d xz and d yz orbitals will be lower in energy than the d z 2 and d x 2-y 2, which will have higher energy, because the ...
Solid State ChemiStry and itS appliCation 2014 Anthony R. West

Construct A Crystal Field Splitting Diagram For Each Of The Following And State How Many Unpaired Homeworklib
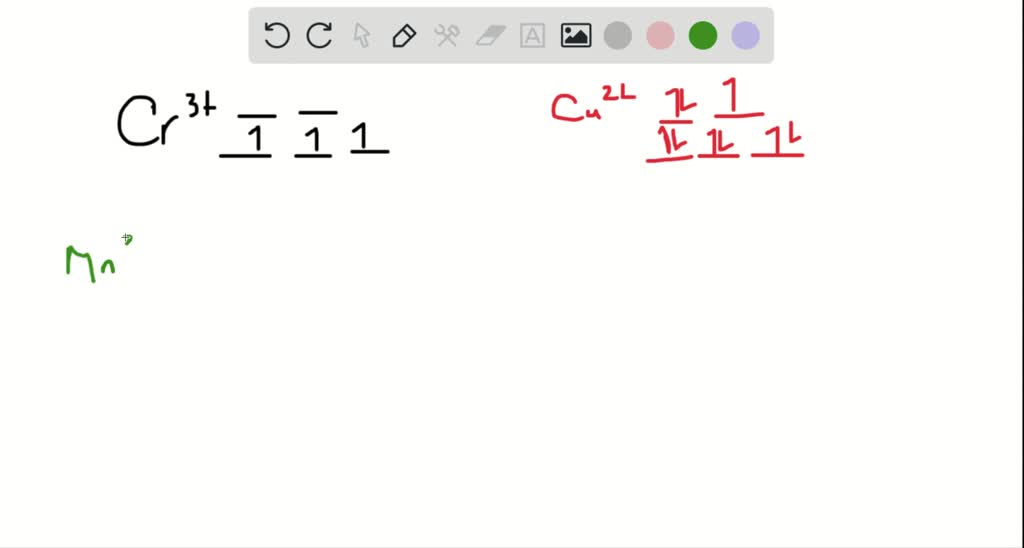
Solved Construct The Octahedral Crystal Field Splitting Diagram For The Metal In Each Species You Are Currently In A Labeling Module Turn Off Browse Mode Or Quick Nav Tab To Items Space Or Enter
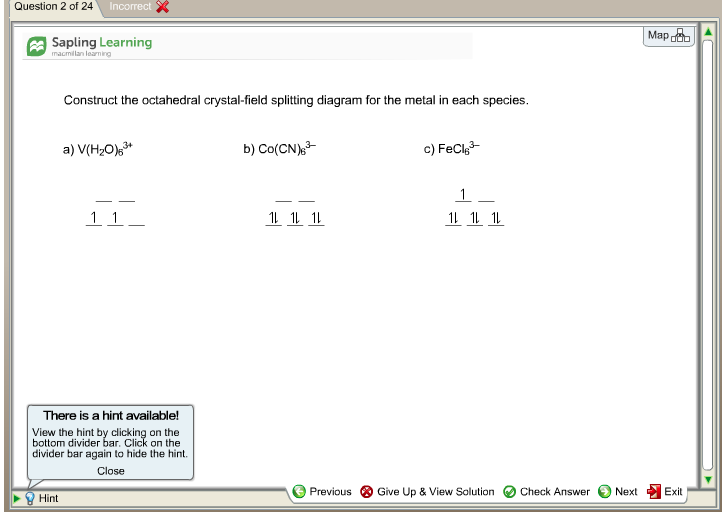
Construct The Octahedral Crystal Field Splitting Diagram For The Metal In Each Species Wiring Site Resource
24 7 Crystal Field Theory Splitting Patterns For Octahedral Tetrahedral And Square Planar High And Low Spin Spectrochemical Series And Estimating Delta Chemistry Libretexts

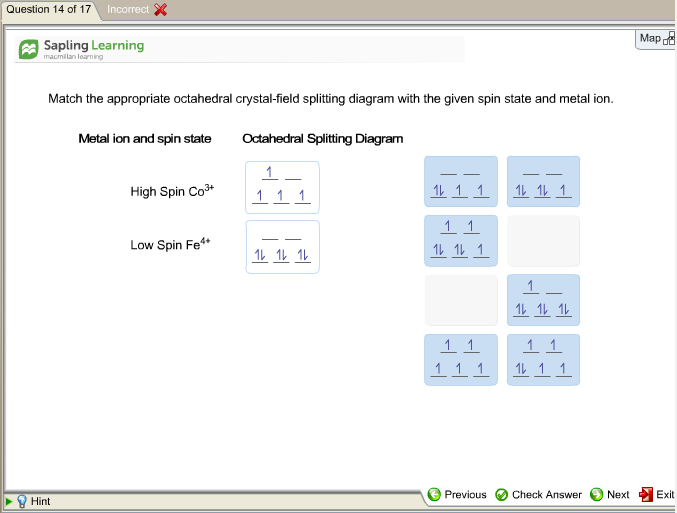
Match The Appropriate Octahedral Crystal Field Splitting Diagram With The Given Spin State And Metal Ion Metal Homeworklib

Match The Appropriate Octahedral Crystal Field Splitting Diagram With The Given Spin State And Metal Ion Metal Homeworklib

















0 Response to "42 construct the octahedral crystal-field splitting diagram"
Post a Comment