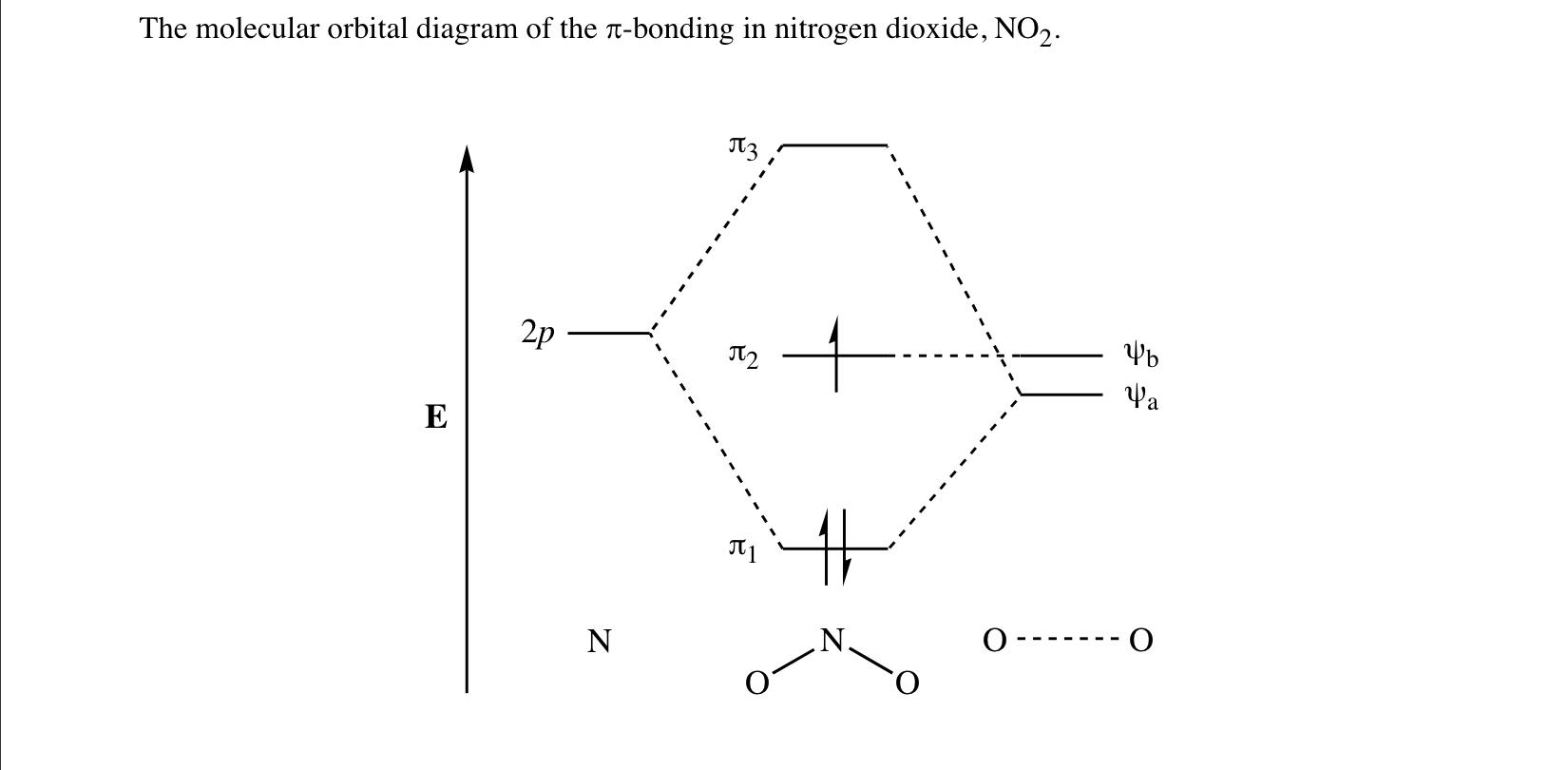
42 no2- molecular orbital diagram
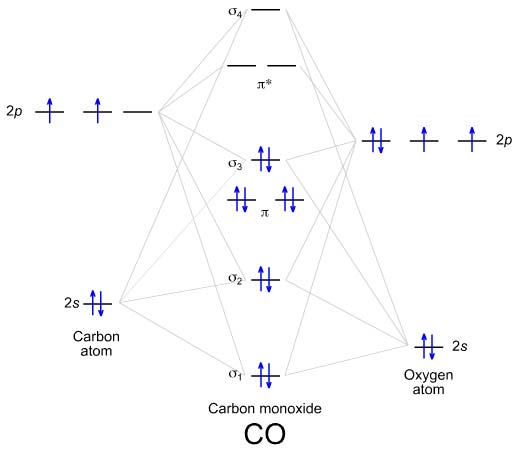
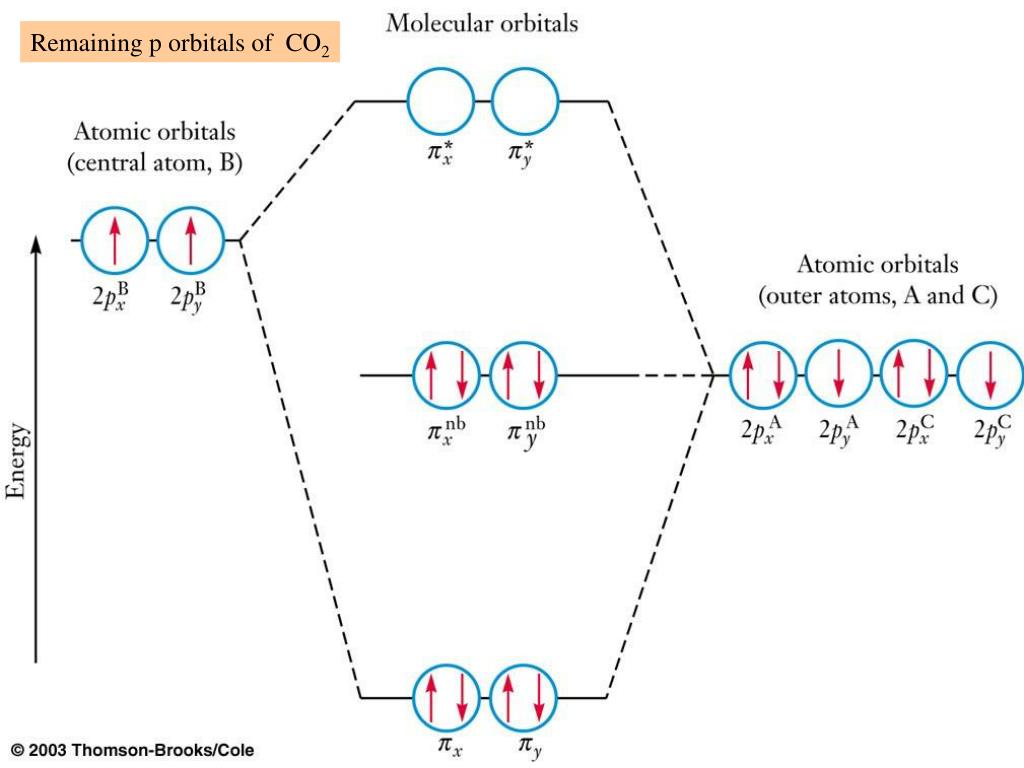
9 Feb 2018 · 1 answerYou've seen the molecular orbital (MO) diagram of CO2 : Inorganic Chemistry, Miessler, Fischer, and Tarr, pg. 148. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
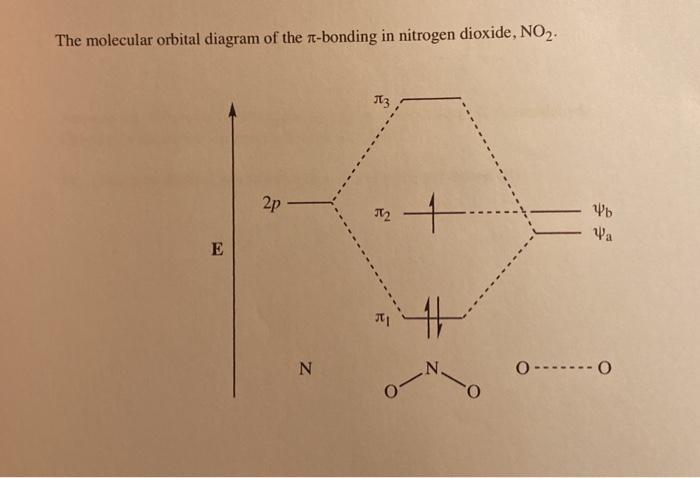
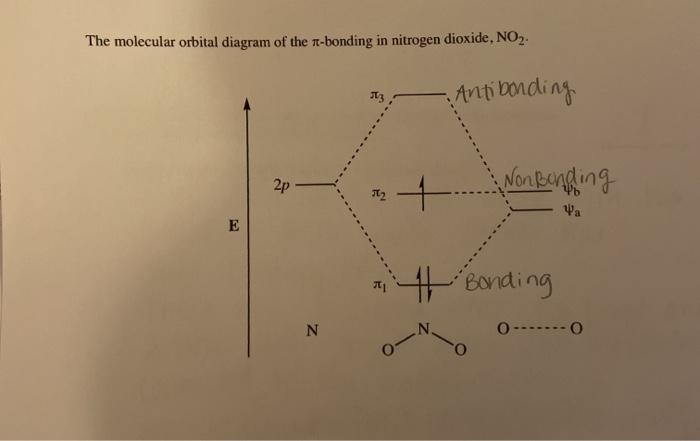
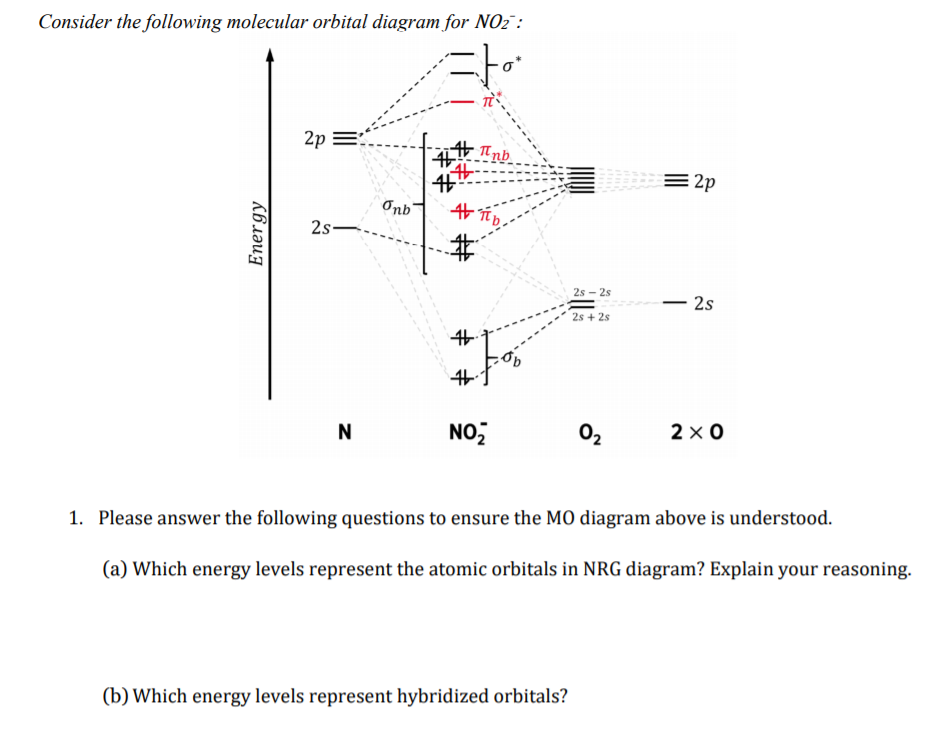
The ion $\ce{NO2^+}$ is linear, $\ce{NO2}$ is bent and so is $\ce{NO2^-}$ with an angle of 101 degrees. To understand these differences it is necessary to combine the p orbitals on each atom to form various $\pi$ orbitals and then consider how these orbitals change in energy as the molecule bends. To do this is quite easy but working out what ...

No2- molecular orbital diagram
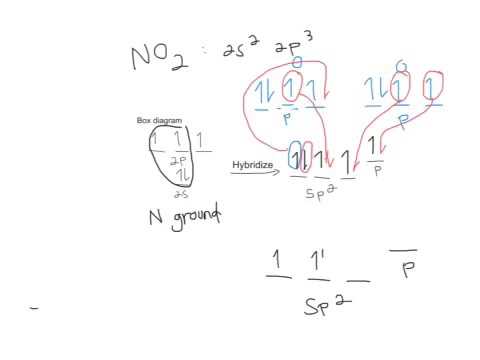
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. Compare the bond order in H 2 + and H 2 using the molecular orbital energy diagram for ... Sigma pi bond formation Orbital overlap concept ncert NO2-: Central atom = N Type of hybrid orbitals on the nitrogen atom = Number of hybrid orbitals used in overlap with atomic orbitals = Number of hybrid orbitals accommodating unshared electrons Question : Answer the following questions about the hybrid orbitals on the nitrogen atom in the NO2- and NO3- molecular ions.
No2- molecular orbital diagram. This problem has been solved! See the answer. See the answer See the answer done loading. NO2+ molecular orbital diagram. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (1 rating) Answer to: Draw and explain the molecular orbital diagram for NO2. By signing up, you'll get thousands of step-by-step solutions to your homework... Answer: I think you can safely assume to start off with the molecular orbital diagram of the Nitrite anion (NO₂¯) and then remove an electron from it: What will be the molecular orbital diagram for nitrite ion? The outcome, i.e. the molecular orbital diagram for Nitrogen dioxide NO₂, should loo... Sketch the MO diagram for C4H6 (1,3-butadiene, CH2=CH–CH=CH2). Page 3. 3. 3. Assume light is absorbed by NO2. − to create the excited molecule (NO2.5 pages
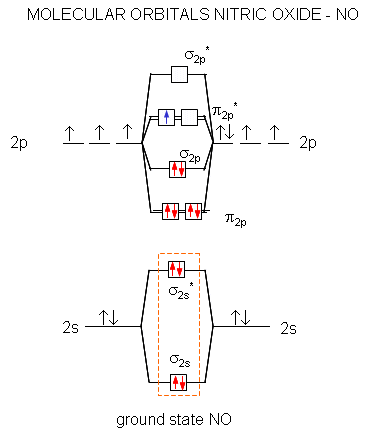
Nitrogen forms a uniquely large number of oxides [1], including amongst others nitrous oxide (N2O), a potent greenhouse gas, nitric oxide (NO), and nitrogen ... Let's look at the molecular orbital diagram of ozone. We'll use the hybrid orbital approximation. ... Nitrogen dioxide has one fewer electron than ozone. Nitrogen is a group 5A element having 5 valence electrons and oxygen has 6 valence electrons (Group 6A). The ion has a +2 charge, so it is missing 2 electrons.1 answer · Top answer: Hey there! To calculate bond order of NO2+ ion and predict other properties, we will start by drawing the molecular orbital diagram for the NO2+ ion.NO2+ ... Nitrogen Dioxide. Molecular orbitals in NO2. Will the molecule be linear or bent? Click on a color picture to watch the geometry change from linear to bent.
No2 Molecular orbital Diagram. molecular orbital theory structure of no2 pound the electron population of this orbital is see table vi 0 53 on the n atom 0 16 2s 0 37 2pz 0 24 on each o atom 0 24 pz experimentally oxides and oxyions of the non metals part ii c02 and no2 of the chemical society 1962 2873 2880 collects values for the partition of unpaired electron density among n and o atomic ... Lewis Structure of NO2. A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen. Let us look at the periodic table. Nitrogen belongs to group 15 ( or group 5) and has an atomic number of 7, therefore has a valency of 5. Oxygen belongs to group 16 ( or group 6) and has an atomic number of 8, therefore a valency of 6. NO2-: Central atom = N Type of hybrid orbitals on the nitrogen atom = Number of hybrid orbitals used in overlap with atomic orbitals = Number of hybrid orbitals accommodating unshared electrons Question : Answer the following questions about the hybrid orbitals on the nitrogen atom in the NO2- and NO3- molecular ions. Sigma pi bond formation Orbital overlap concept ncert
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. Compare the bond order in H 2 + and H 2 using the molecular orbital energy diagram for ...
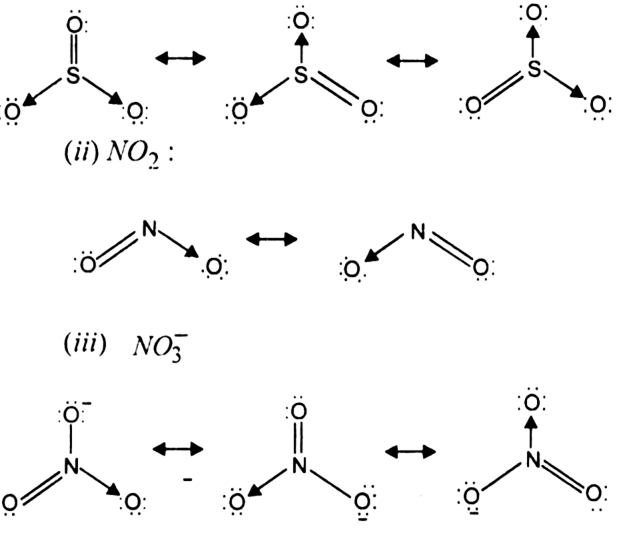
Write The Resonance Structures For So3 No2 And From Chemistry Chemical Bonding And Molecular Structure Class 11 Haryana Board English Medium

Dft Insights Into The Electronic Properties And Adsorption Of No2 On Metal Doped Carbon Nanotubes For Gas Sensing Applications New Journal Of Chemistry Rsc Publishing

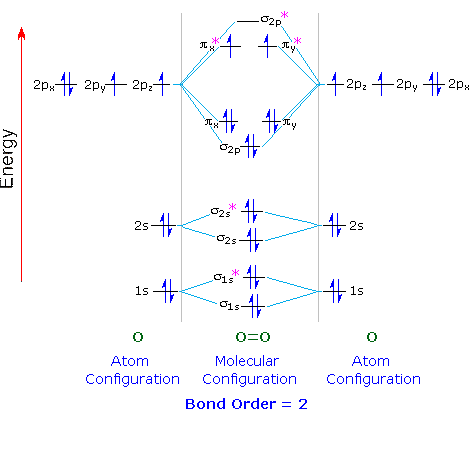
Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11














0 Response to "42 no2- molecular orbital diagram"
Post a Comment