42 potential energy diagram catalyst
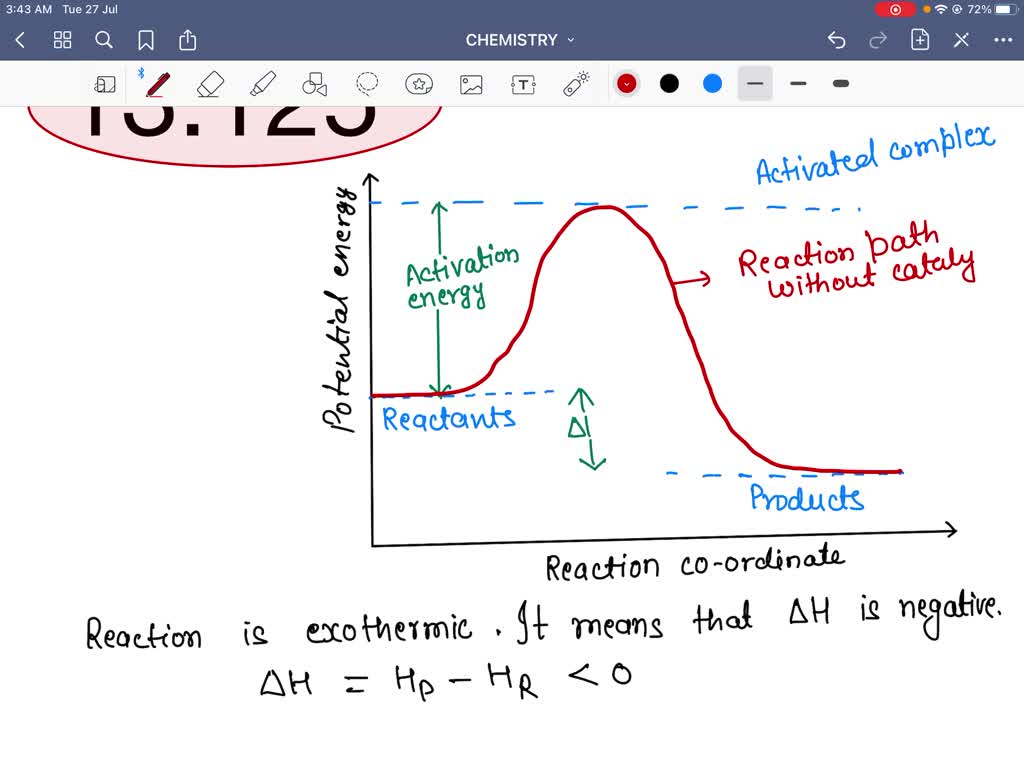
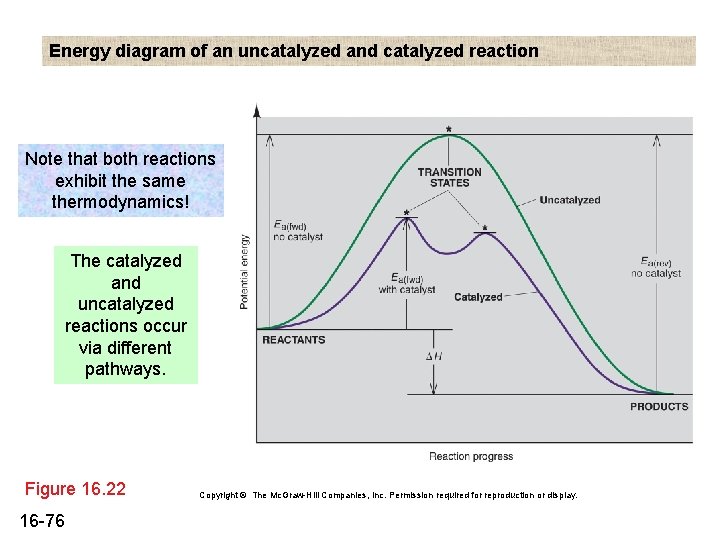
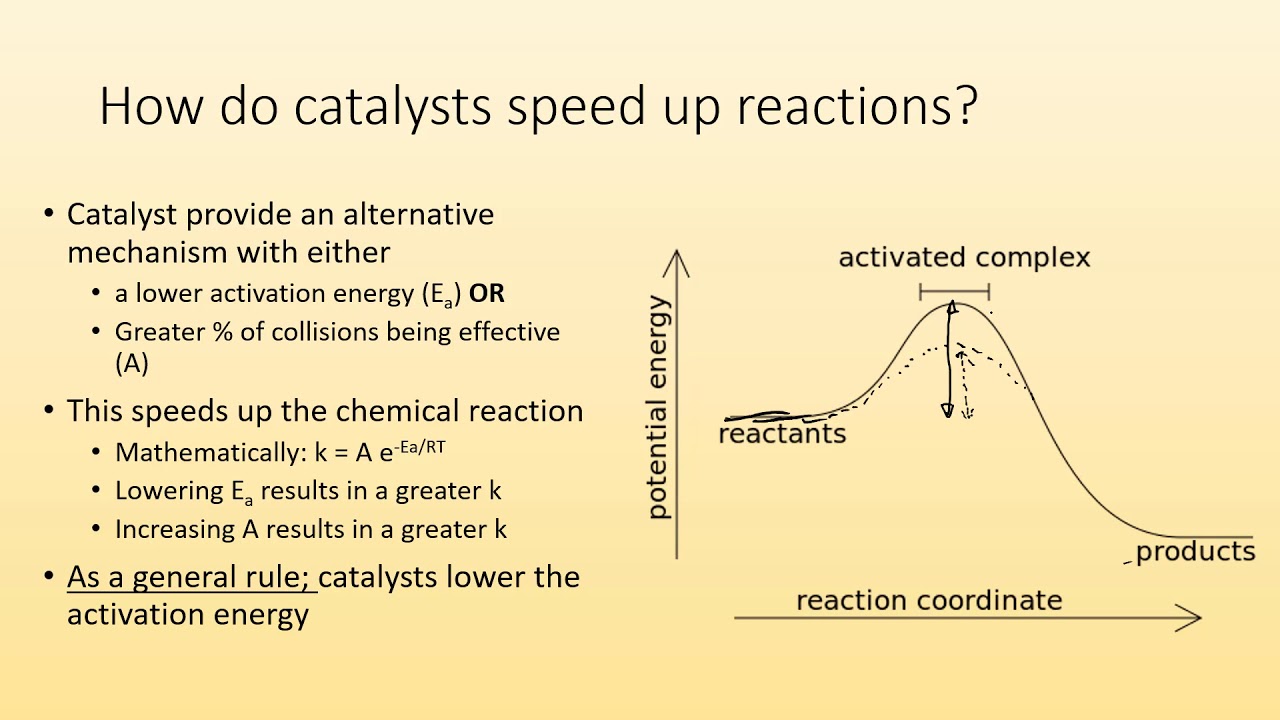
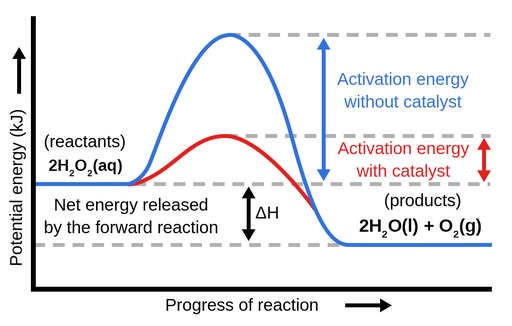
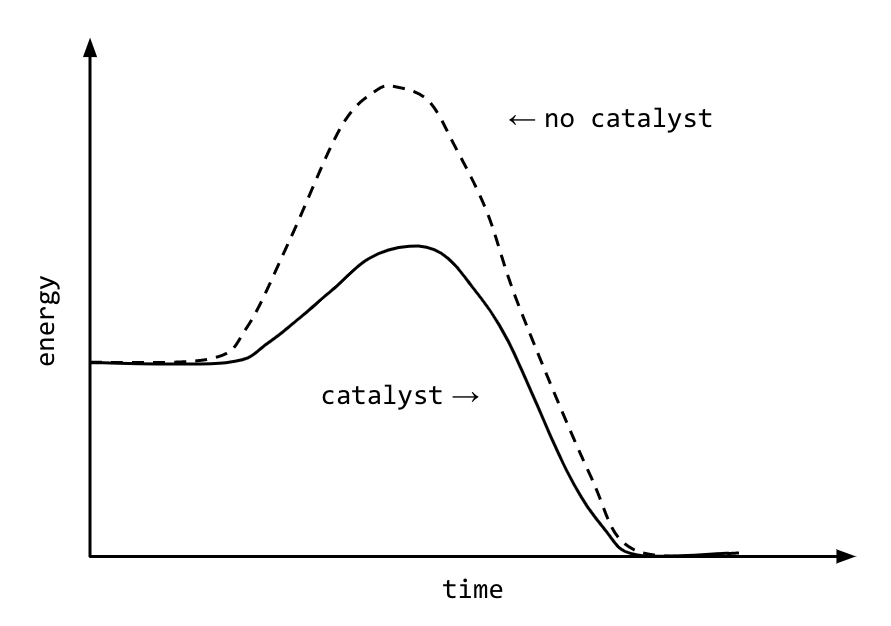
A catalyst, or enzyme, works with a substrate to decrease the amount of initial energy required to perform a specific chemical opperation, speeding the reaction up. Enzymes also work to increase ... Figure 6 shows the generic potential energy diagram with a catalyst and noncatalyst in a hypothetical exothermic chemical reaction. Catalyst works by providing ...
Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams List examples of catalysis in natural and industrial processes Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst , a substance that can increase the reaction rate without being ...

Potential energy diagram catalyst
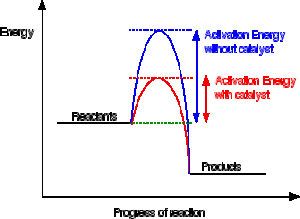
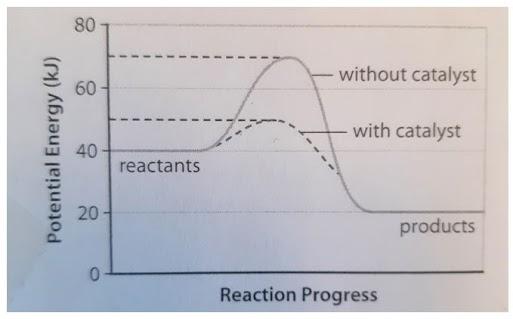
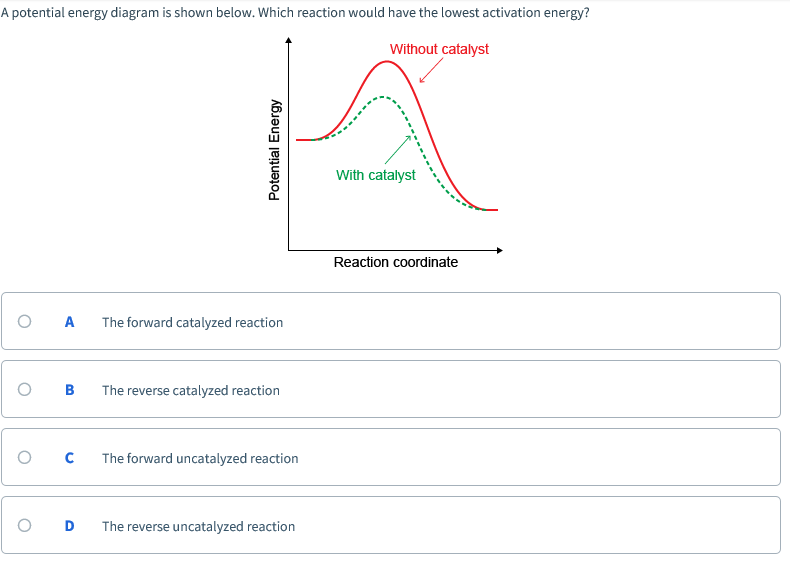
Potential energy barriers for catalyzed and uncatalyzed reactions; The potential energy diagram compares the potential energy barriers for the catalysed and uncatalysed reactions. The barrier for uncatalysed reaction (E a) is larger than that for the same reaction in the presence of a catalyst E a. Potential Energy Diagram The graph shows a reaction rate with and without the use of a catalyst. Summary. A catalyst is a substance that increases the rate of a chemical reaction. A catalyst provides an alternate pathway for the reaction that has a lower activation energy. When activation energy is lower, more reactant particles have enough ... An Energy Profile is also referred to as an Energy Diagram or as a Potential Energy Diagram. An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change , ΔH, is the amount of energy absorbed or released by a chemical reaction.
Potential energy diagram catalyst. 4 Sept 2021 ... Figure 12.7.2: This potential energy diagram shows the effect of a catalyst on the activation energy. The catalyst provides a different reaction ... 36.A potential energy diagram for a chemical reaction is shown below. On this diagram, draw a curve to show how the potential energy diagram will change when a catalyst is added to the reaction. Name Unit 10 Practice Test 37. Base your answer to the following question on the information below. potential energy diagrams. The total potential energy of all the reactants in the reaction is illustrated at the left of the diagram, the total potential energy of the products is at the right side, and the activation energy change or changes are illustrated in between. On the grids below, plot energy diagrams illustrating the reactions ... Answer: The overall diagram will depend on whether the reaction is endothermic (final H is higher then initial H) or exothermic (final H is lower than initial H) BUT: Catalysed reactions lower the activation energy - the hump that needs to be overcome for the reaction to proceed. (Catalysts ofte...
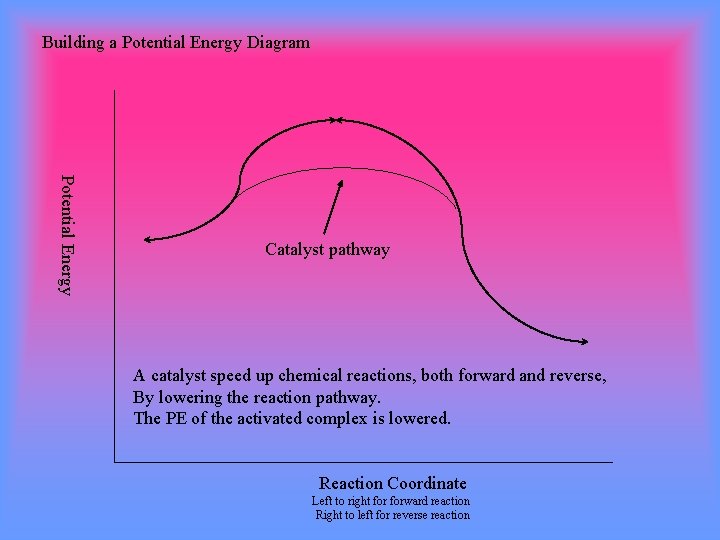
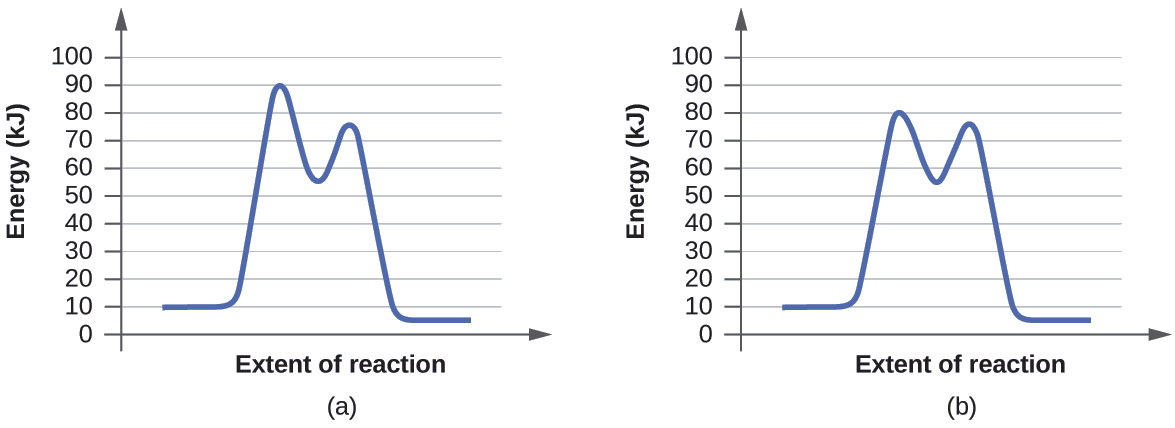
Potential energy. Energy Diagrams for Catalyzed and Uncatalyzed Reactions. Page 2. Page 3. Page 4. Enzymatic Catalysis of a Reaction between Two Substrates. Page 5. Enthalpy profile for an non-catalysed reaction, last page a typical, non- catalysed reaction can be represented by means of a potential energy diagram. 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process. This graph compares potential energy diagrams for a single-step reaction in the presence and absence of a catalyst. The only effect of the catalyst is to lower the activation energy of the reaction. The catalyst does not affect the energy of the reactants or products (and thus does not affect ΔE). Reaction coordinate diagrams are derived from the corresponding potential energy surface (PES), which are used in computational chemistry to model chemical ...
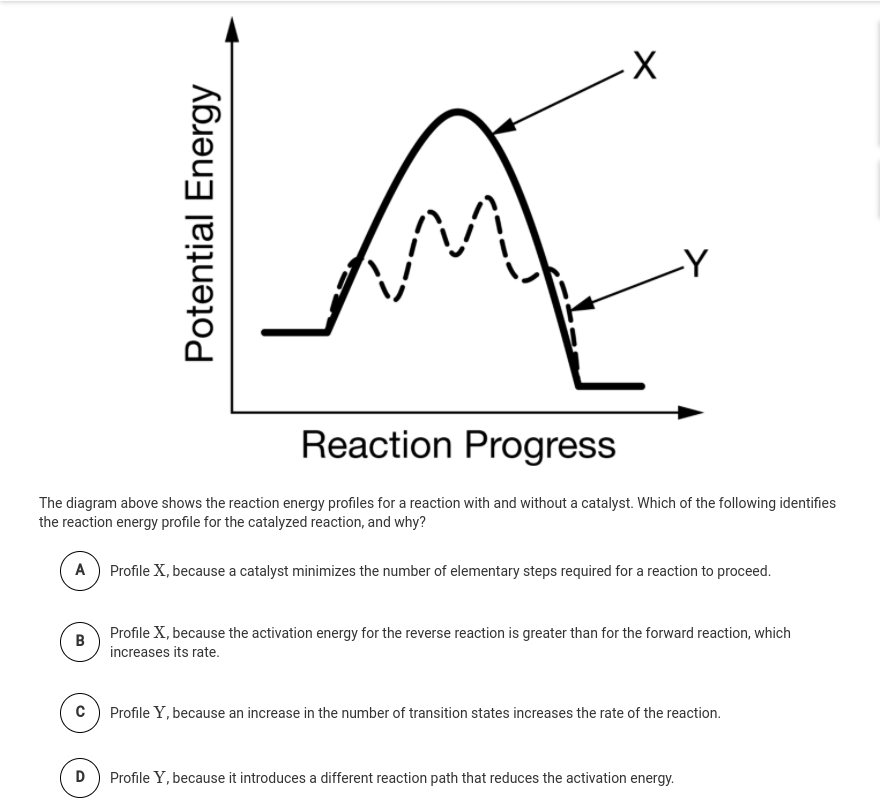
A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values. This potential energy diagram shows the effect of a catalyst on the activation energy. The catalyst provides a different reaction path with a lower activation energy. As shown, the catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two ... Potential Energy Diagram Worksheet ANSWERS 1. Which of the letters a-f in the diagram represents the potential ... A catalyst changes the reaction mechanism, in the process lowering the activation energy. 5. Name 4 things that will speed up or slow down a chemical reaction. Potential energy diagrams. ... catalyst provides an alternative reaction pathway which involves less energy and so the catalyst lowers the activation energy. The use of a catalyst does not ...
Diagram 2 depending on the values but looks quite small. To be spontaneous needs to be ≤ 20kJ 2. Draw a potential energy (E p ) diagram for a reaction in which ∆H = 80 kJ/mol and E a = +28kJ/mol. Label the axes, activation energy, ∆H, site of the activated
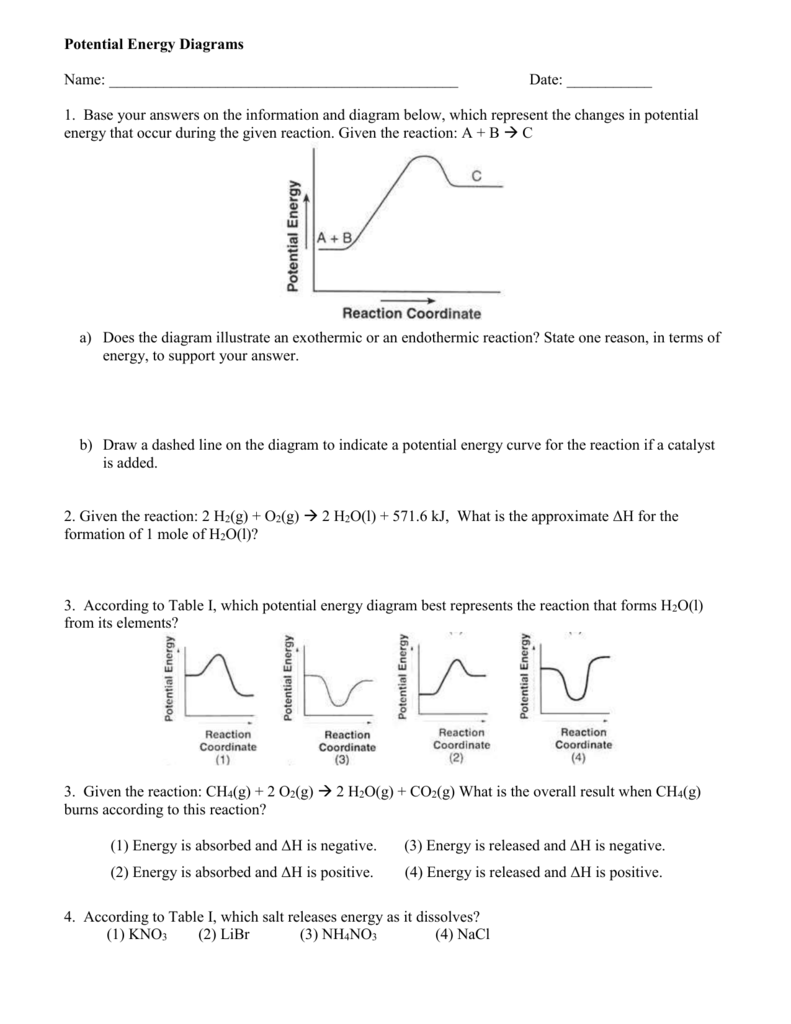
Does this potential energy diagram represent an exothermic or an endothermic reaction? [Explain whv.] According to the diagram, is the potential eneoy of the products greater than, less than, or equal to the potential energy of the reactants? Draw an alTOW on the diagram above to represent the activation energy for the forward reaction. Label the
This potential energy diagram shows the effect of a catalyst on the activation energy. The catalyst provides a different reaction path with a lower activation energy. As shown, the catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two ...
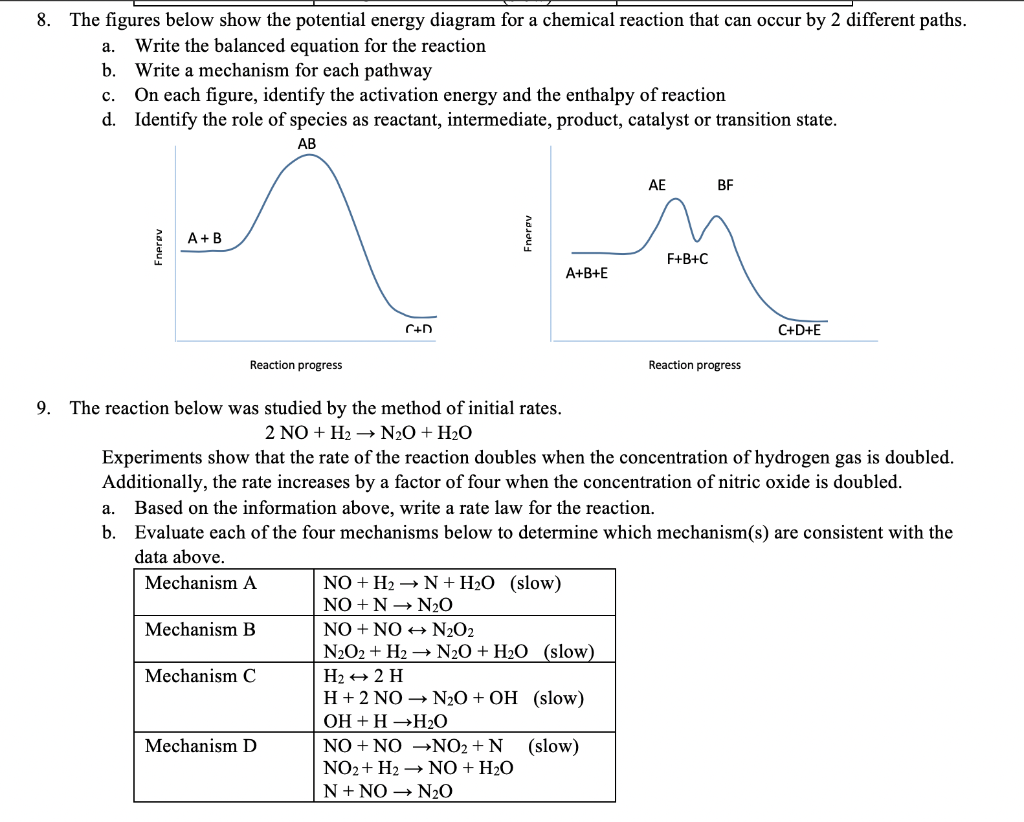
Unit 9 Kinetics SA Review Page 3 10.A potential energy diagram for a chemical reaction is shown below. On this diagram, draw a curve to show how the potential energy diagram will change when a catalyst is added to the reaction.
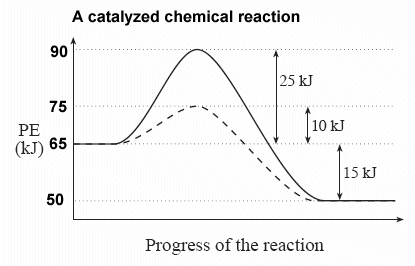
S(s) + 02(g) S02(g) + energy Which diagram best represents the potential energy changes for this reaction? 12. Given the potential energy diagram for a reversib chemical reaction: Reaction Coordinate Each interval on the axis labeled "Potential Energy (kJ/mol)" represents 10. kilojoules per mole. Wha is the activation energy of the forward ...
potential energy A B P Figure 1.2. Potential energy diagram of a heterogeneous catalytic reaction, with gaseous reactants and products and a solid catalyst. Note that the uncatalyzed reaction has to overcome a substantial energy barrier, whereas the barriers in the catalytic route are much lower. The energy diagram of Fig. 1.2 illustrates ...
The potential energy diagram of a chemical reaction is shown. Interval B represents the A. potential energy of the products C. activation energy B. potential energy of the reactants D. activated complex 30. The combustion of propane is best described as an A. endothermic chemical change C. exothermic chemical change
The potential energy diagram below shows the reaction X + Y → Z. When a catalyst is added to the reaction, it will change the value of 1) 1 and 2 3) 2 and 3 2) 1 and 3 4) 3 and 4 17. According to Table I, the least amount of energy would be evolved by the formation of one mole of
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
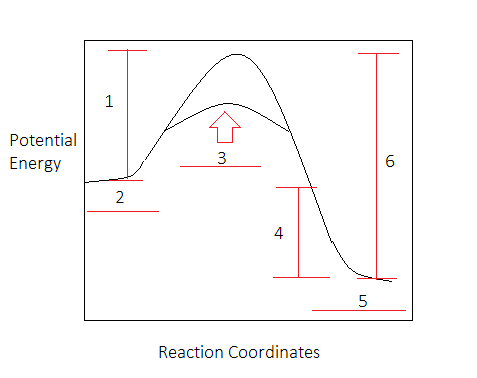
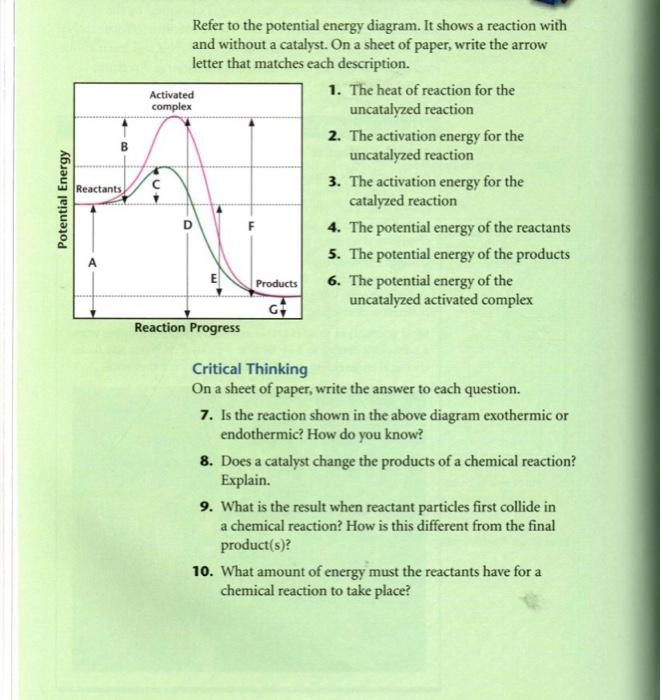
7. What letter represents the potential energy of the activated complex? 8. Is the reverse reaction endo or exothermic? 9. If a catalyst were added, what letter(s) would change? a e c a and c 12. POTENTIAL ENERGY DIAGRAM WS 1. Which of the letters a-f in the diagram represents the potential energy of the products? _____ 2.
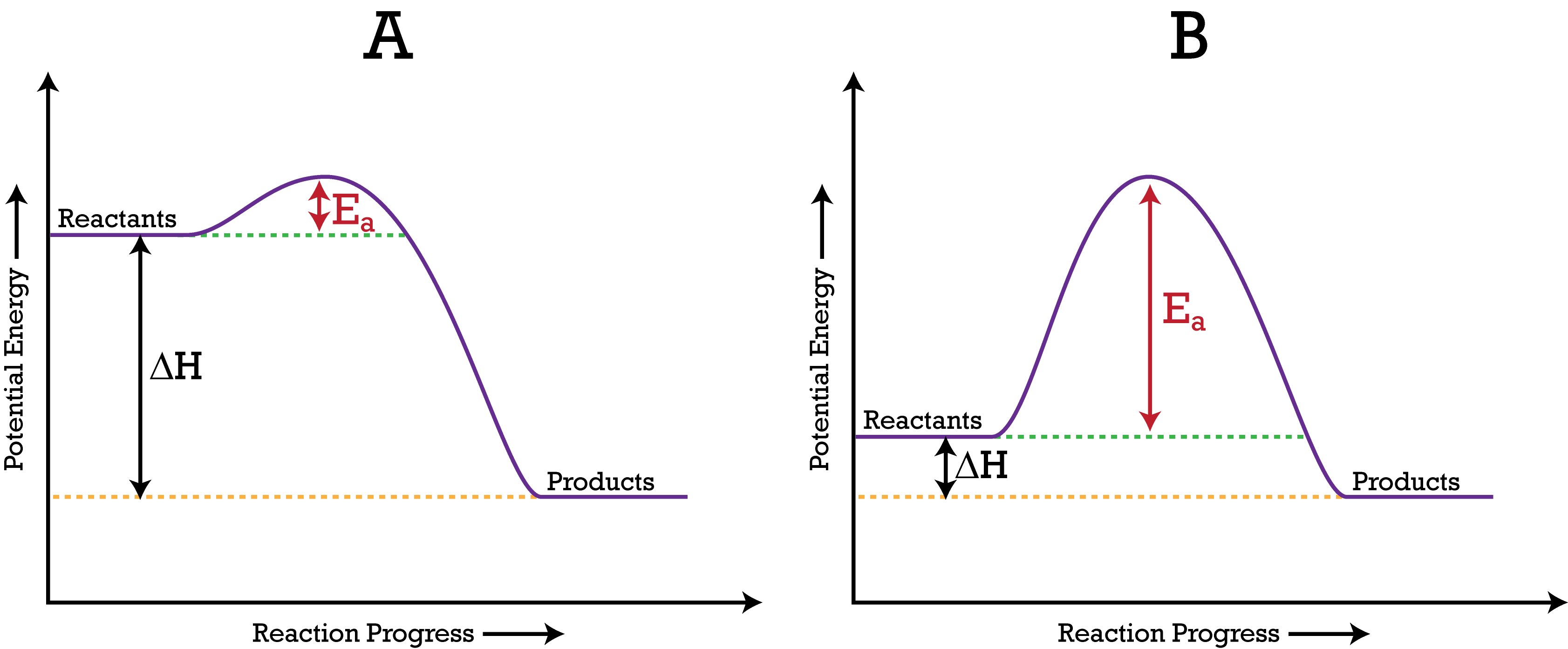
An energy diagram can be defined as a diagram showing the relative potential energies of reactants, transition states, and products as a reaction progresses with time. The activation energy is the difference in the energy between the transition state and the reactants. It's depicted with a red arrow.
Energy diagrams are useful to illustrate the effect of a catalyst on reaction rates. Catalysts decrease the activation energy required for a reaction to proceed ...

Figure 10 Hydrogenation Of Styrene Oxide To 2 Phenylethanol Over Nanocrystalline Ni Prepared By Ethylene Glycol Reduction Method
Exercise 2 : State whether the reactions below are fast or slow, and exothermic or endothermic. a) b) PE time If a catalyst is used, the reaction proceeds much ...
Adding a catalyst has exactly this effect of shifting the activation energy. A catalyst provides an alternative route for the reaction. That alternative route has a lower activation energy. Showing this on an energy profile: A word of caution! Be very careful if you are asked about this in an exam. The correct form of words is
Activation energy is the energy required to "jump start" the reaction. Its the energy added to initiate reaction. The energy requirements can be reduced with the addition of a catalyst. Catalyst -substance that speeds up a chemical reaction without being consumed. Ex. Enzymes are a biological catalyst speeding up biochemical processes.
Sketch the potential energy diagram for an exothermic reaction with a catalyst added.
What letter represents the potential energy of the activated complex? 8. Is the reverse reaction endothermic or exothermic? endothermic. 9. If a catalyst were ...
An Energy Profile is also referred to as an Energy Diagram or as a Potential Energy Diagram. An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change , ΔH, is the amount of energy absorbed or released by a chemical reaction.
Potential Energy Diagram The graph shows a reaction rate with and without the use of a catalyst. Summary. A catalyst is a substance that increases the rate of a chemical reaction. A catalyst provides an alternate pathway for the reaction that has a lower activation energy. When activation energy is lower, more reactant particles have enough ...
Potential energy barriers for catalyzed and uncatalyzed reactions; The potential energy diagram compares the potential energy barriers for the catalysed and uncatalysed reactions. The barrier for uncatalysed reaction (E a) is larger than that for the same reaction in the presence of a catalyst E a.

Draw A Graph Of Potential Energy V S Reaction Coordinate Showing The Effect Of A Catalyst On Activation Energy

Explain With The Help Of A Potential Energy Diagram That The Catalyst Increases The Rate Of The Reaction Chemistry Shaalaa Com

Draw An Energy Diagram For An Endothermic Reaction Without A Catalyst Use A Solid Line And With A Brainly Com
Solved Draw A Potential Energy Diagram For This Reaction And Draw A Second Line To Represent How The Reaction Would Be If A Catalyst Was Used 6co2 G 6h2o I 2803kj C6h12o6 S 6o2 G













0 Response to "42 potential energy diagram catalyst"
Post a Comment