36 oh molecular orbital diagram
MO diagrams of Heteronuclear Diatomic Molecules |PART-1 | OH-,HF, CN- |By ved Sir | Chem academyhttps://www.youtube.com/channel/UCNPYcBEfxHqTwkxLEw1sHXwThis ...
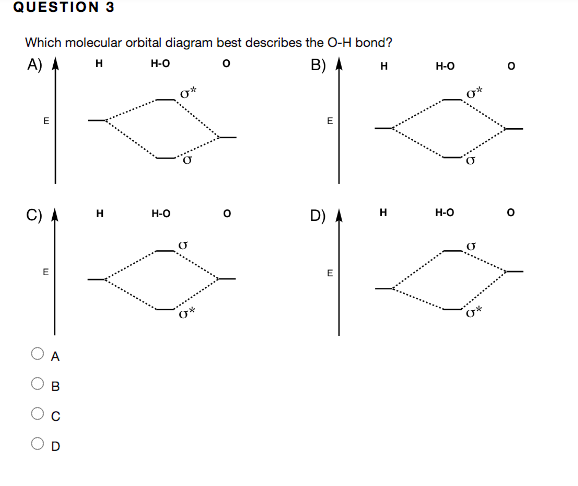
Dec 30, 2019 · Bookmark this question. Show activity on this post. I would like to understand how to create a molecular orbital diagram for the hydroxide ion from scratch. This includes understanding the shape of the molecular orbital. Here is an attempt I have come up with: Where the top MO is sigma* and the bottom is simply sigma. But this makes no sense.
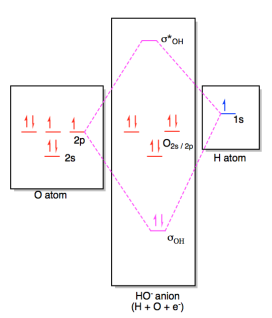
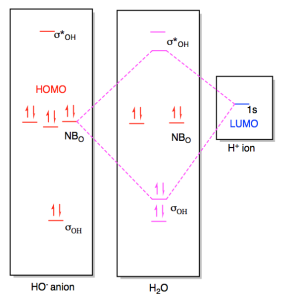
Oh- molecular orbital diagram FIG. 1: Two molecular orbital s of water that have bonding ˙ OH character, 2a 1 and 1b 1. Their combined contribution leads to 2 single OH bonds: (2a 1)2(1b 1)2!(˙ OH1)2(˙ OH2)2 ˙ orbital s are oriented along the bonds, whereas ˇ- orbital s are oriented perpendicular to the molecular plane.
Oh molecular orbital diagram
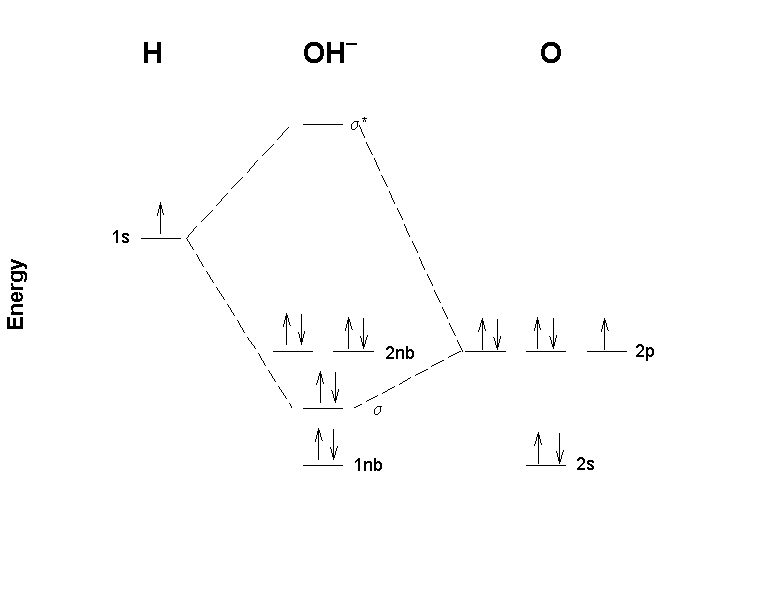
Energy level diagram for the molecular orbitals of OH ). H and O atom orbitals, which combine to form the molecular orbitals, are on the left and right side of the figure, respectively. Source...
Where is the energy difference (Between which orbitals) that corresponds to o (10Dq)? Label o on the MO diagram. What type of orbitals (bonding, non-bonding, antibonding) are the "crystal field" orbitals? Explain why. Calculate the o for [Co(NH3)6]3+, then determine if it is low- or high-spin. Fill in the MO diagram accordingly.
d orbitals •l = 2, so there are 2l + 1 = 5 d-orbitals per shell, enough room for 10 electrons. •This is why there are 10 elements in each row of the d-block. σ‐MOs for Octahedral Complexes 1. Point group Oh 2. The six ligands can interact with the metal in a sigma or pi fashion.
Oh molecular orbital diagram.
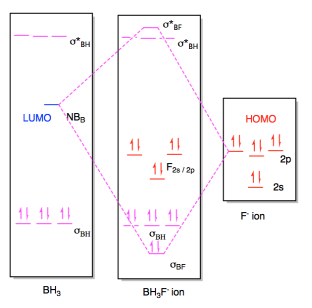
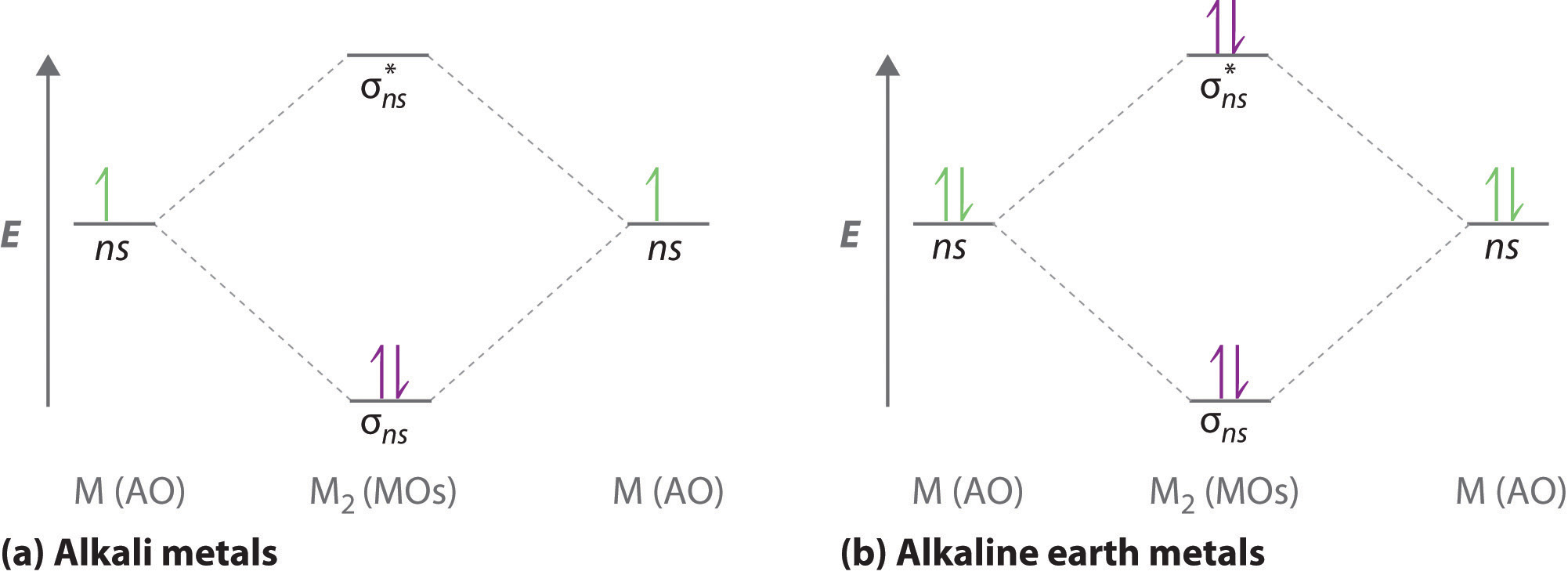
Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
Molecules Free Full Text Oh Molecular Orbital Diagram. Chemistry Archive November 13 2016 Oh Molecular Orbital. Molecular Orbital Energy Level Diagram Atkinsjewelry. Construct The Orbital Diagram For As Wiring Diagram Database. Ppt Lecture 17 Molecular Orbital Theory 1 Molecular.
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
Molecular orbital diagram for the Fe3*Mn2*O,o clus-ter in the (a) ferromagnetic and (b) antiferromagnetic configu-rations. Orbitals indicated with a dashed line are unoccupied. Note that the orbital energies correspond to "orbital electronega-tivities" . Dec 05, · Upload failed.
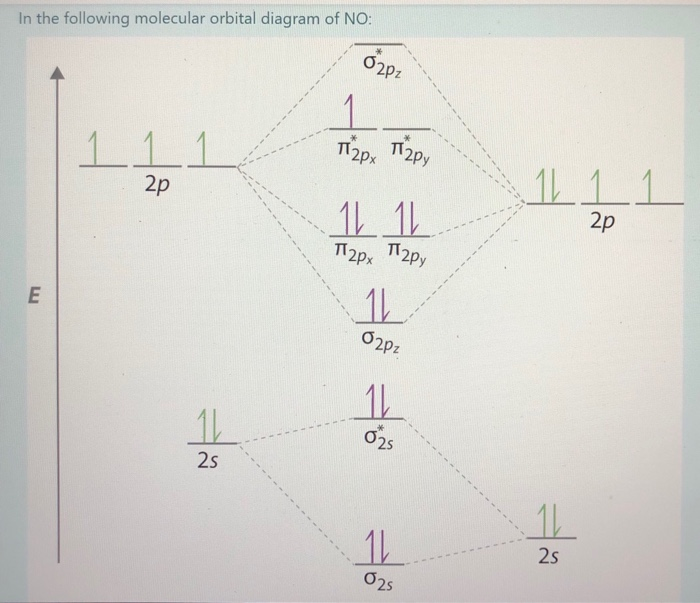
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Hydroxide | HO- | CID 961 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards ...
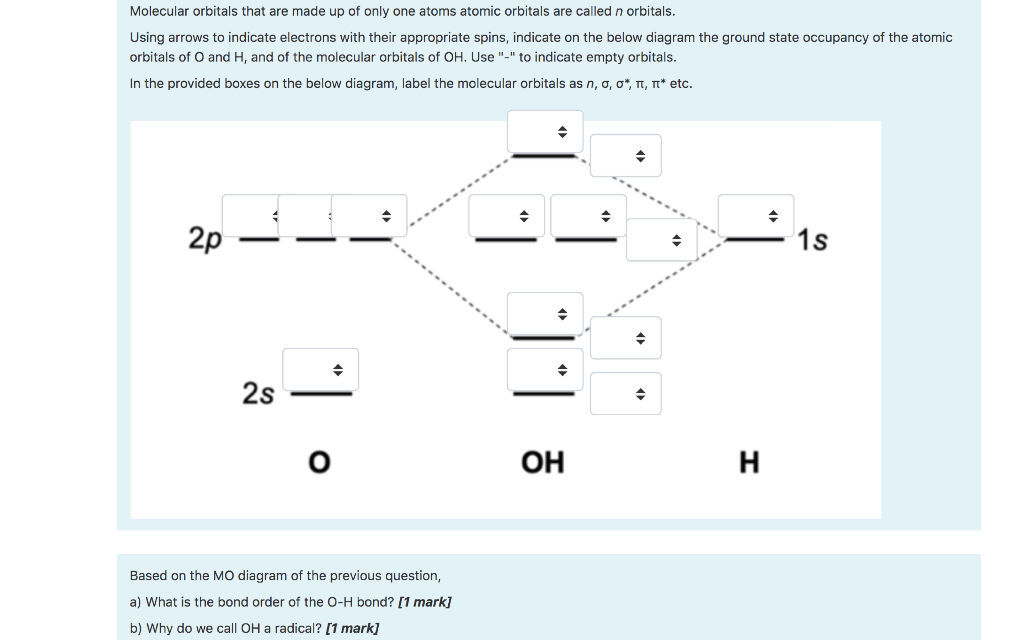
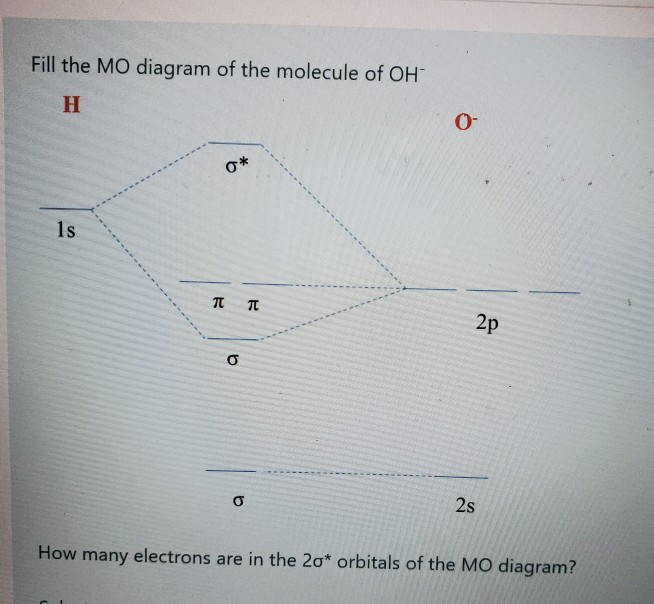
Core orbitals are omitted. Marks 8 Using arrows to indicate electrons with their appropriate spin, indicate on the above diagram the ground state occupancy of the atomic orbitals of O and H, and of the molecular orbitals of OH. In the provided boxes on the above diagram, label the molecular orbitals as n, σ, σ∗, π, π∗, etc.
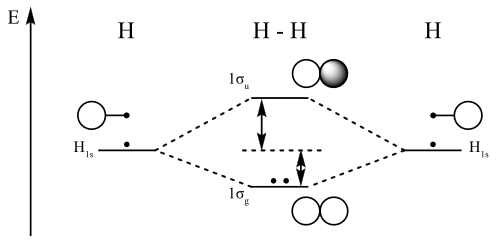
Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
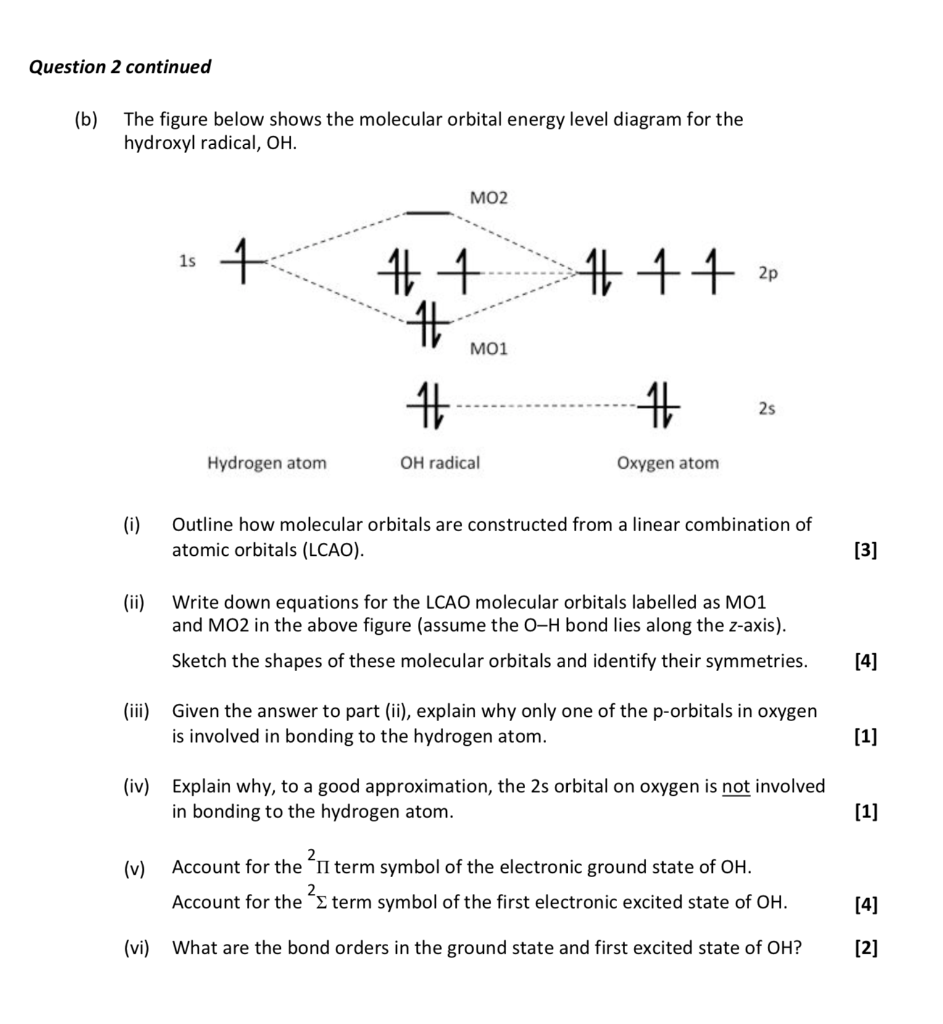
Nov 04, 2008 · Molecular orbital of the hydroxyl radical with unpaired electron Skeletal formulae of 1-hydroxy-2(1H)-pyridinethione and its tautomer The hydroxyl radical, OH, is the neutral form of the hydroxide ion. Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
Molecular Orbitals for Water (H 2 O) The five occupied and the lowest three unoccupied molecular orbitals of the isolated molecule (1a 1) 2(2a 1) 2(1b 2) 2(3a 1) 2(1b 1) 2 were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set (experimental data is given in [1289]). They are set out with the lowest
This photo about: Oh Molecular orbital Diagram, entitled as Hf Molecular Orbital Diagram - Orbital Diagram For Fluorine Awesome Oh Molecular Orbital Diagram - also describes Hf Molecular Orbital Diagram - Orbital Diagram For Fluorine Awesome and labeled as: ], with resolution 1990px x 1360px
The hydroxyl radical is the diatomic molecule • OH.The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals is produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water.
features of molecular orbital theory for metal complexes are as follows: 1.The atomic orbital of the metal center and of surrounding ligands combine to form new orbitals, known as molecular orbitals. 2.The number of molecular orbitals formed is the same as that of the number of atomic orbitals combined.
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
Oh Molecular orbital Diagram. molecular orbitals for oxonium and hydroxide ions description of the molecular orbitals for oxonium h3o and hydroxide oh ions o oh h faculty of science the university of sydney a molecular orbital diagram of this species is shown below core orbitals molecular orbitals of oh in the provided boxes on the above diagram.
• The energy increase of the e g orbitals and the energy decrease of the t 2g orbitals must be balancedrelative to the energy of the hypotheticalsphericalfield(aka the barycenter).• The energy of each of the two orbitals of the e g set rises by +3/5 o (+6 Dq) while the energy of eachof the three t 2g orbitalsfallsby ‐2/5 o(‐4Dq). • Thisresults inno netenergy changefor the system:
#MOT #BMO #ABMO #HF #CO #NO #CN #OHHello everyoneThis is shivam here To follow me on instagram search - Sshivam898To join telegram group click on the given l...
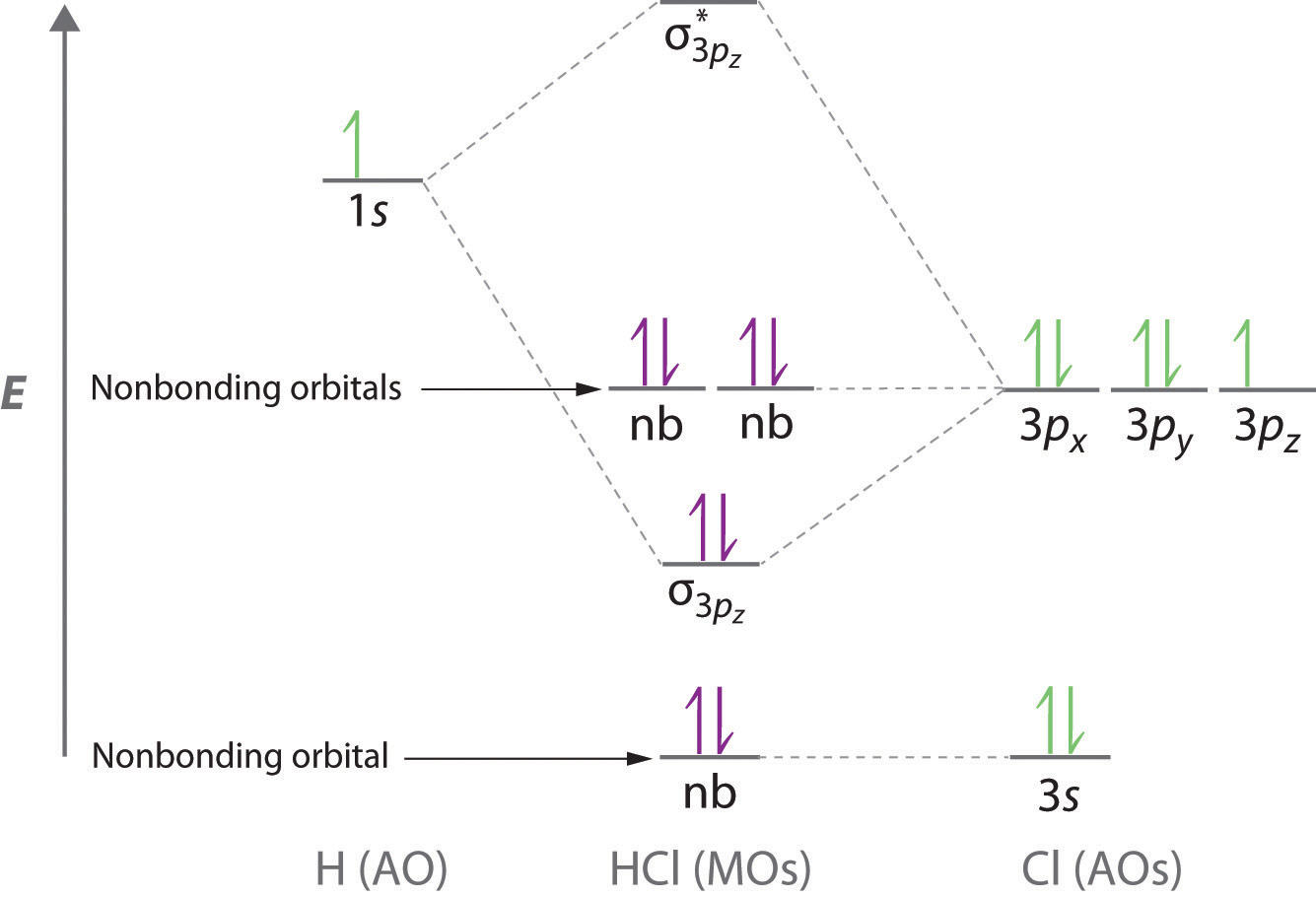
Answer (1 of 2): Here is a useful MO diagram of HCL found on the internet: The Cl electrons residing up to 3s orbital (1s, 2s, 2px,2py,2pz,3s) are largely stabilized than H electron in 1s orbital and therefore they cannot mix and form bond. The 3p electrons of Cl have comparable energy with the ...
homolumo. To get an idea of the tendency of the two species (let's call them molecules A and B) to stick together, we can check the interactions of all pairs of orbitals (their overlap and energy match) and see whether there is a way to lower the total energy from that of the separated molecules. Each molecule has a certain number of (doubly ...
Printable O2 molecular orbital diagrams are available for you to guide your study in the molecular orbital lesson. This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
The reason oh He2 Molecule to not exist can be explained on the basis of 1)MOLECULAR ORBITAL THEORY. He has configuration of 1s2, if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 e's enter the AntiBonding molecular Orbital, thus net effect of the anti bonding and bonding is cancelled.
Molecular Orbital Diagram for the HF Molecule. Interaction occurs between the 1s orbital on hydrogen and the 2p orbital in fluorine causing the formation of a sigma-bonding and a sigma-antibonding molecular orbital, as shown below. Figure 1: Molecular orbitals of HF. (CC BY-SA-NC 2.0 UK: England & Wales License; Nick Greeves).






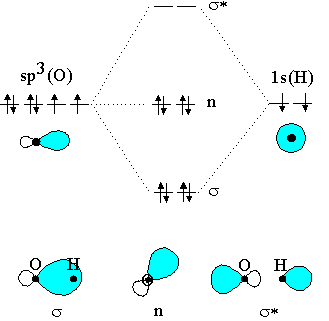








0 Response to "36 oh molecular orbital diagram"
Post a Comment