37 orbital diagram for nickel
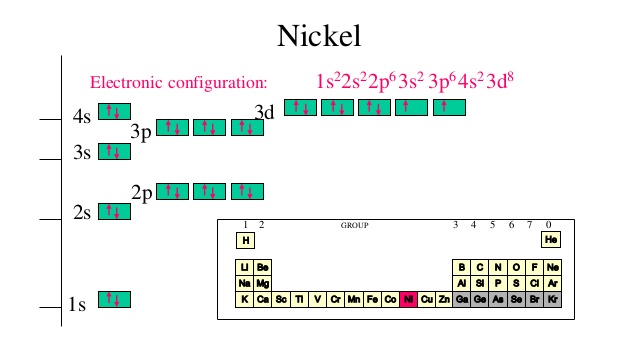
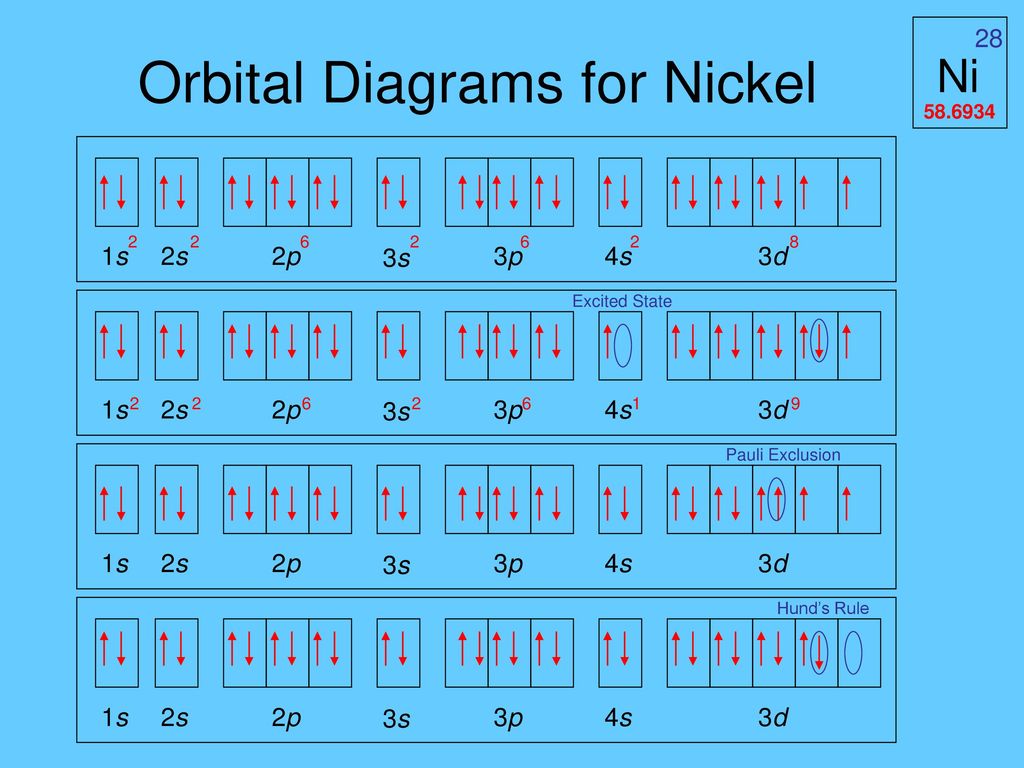
The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d8. In all of the cases, both up and down arrows are filled, with the exception of the 3d shell, where the last two are up ... When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. For each electron shell atom diagram, the element symbol is listed in the nucleus. How to draw an electron configuration diagram · find the element on the periodic table. Lithium atom electron shell diagram element.
The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p64s2 3d8. In all of the cases, both up and down arrows are filled,with the exception of the 3d shell, where the last two are.What is the orbital diagram for nickel? | schematron.orgWhat is the orbital diagram for nickel.
Orbital diagram for nickel
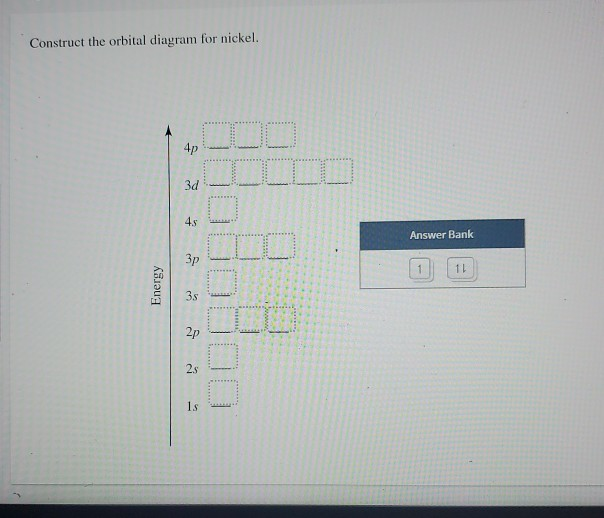
Orbital Diagram For Arsenic. Because the 4p section has 3 orbitals, but Arsenic ends with 4p3. It'll want to leave as few orbitals empty, so you have three arrows pointing up. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org! Transcribed Image Textfrom this Question. Construct the orbital diagram for nickel 1 11 1 4p 1 11 14 1 3d 1 4s Answer Bank 1 1 1 3p 1 1 1L 3s 1 1 1L 2p 1 2s 1s Energy Construct the orbital diagram of the F ion Зр Answer Bank 3s 1 2p 2.s 1s Energy. Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund's Rule: Given sev...
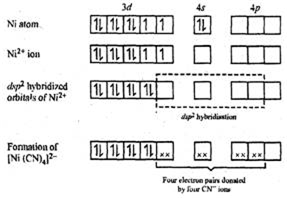
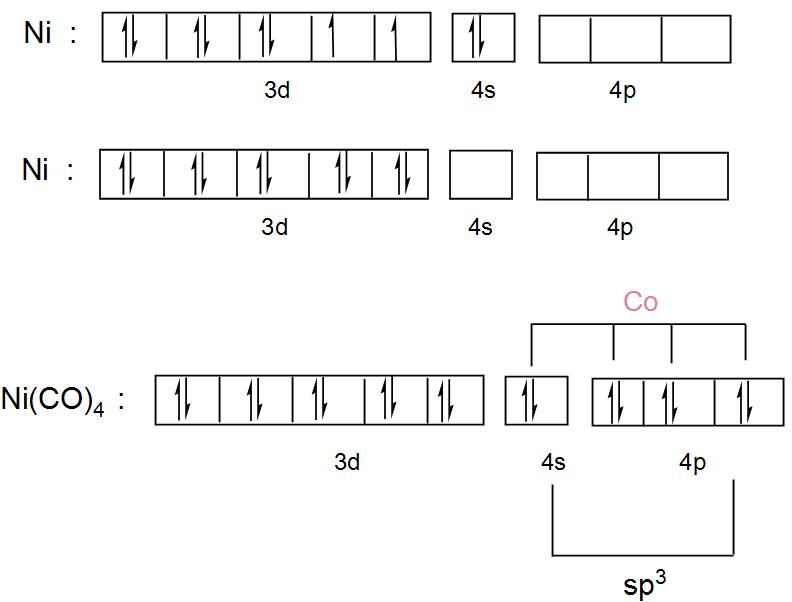
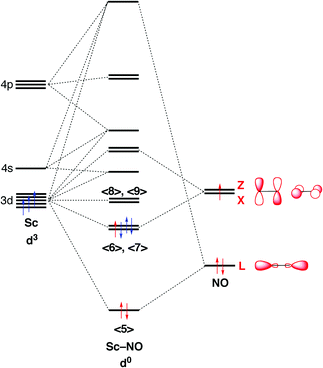
Orbital diagram for nickel. Answer (1 of 2): Instead of acting like an entitled egomaniac who thinks he's the smartest guy in the room like the other guy did, I'll provide an actual answer. Since nickel is a transition element, you have to manually write out its electron configuration and figure out how many electrons the l... Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. The overall molecular orbital energy level diagram for σ-bonding in octahedral complexes can be shown as: Figure 10. The formation of σ-molecular orbitals (bonding, antibonding and non-bonding) in octahedral complexes of transition metals. Buy the complete book with TOC navigation, "Ni"("CO")_4 has nickel in its 0 oxidation state, with electron configuration [Ar] 3d^8 4s^2. So, we call it a d^10 complex in the ligand field. Here is its MO diagram (it is tetrahedral): Here, the 2e and 9t_2 orbitals are what we pick out as the d-orbital splitting diagram with tetrahedral splitting energy Delta_t.
ʻOumuamua is small and not very luminous. It was not seen in STEREO HI-1A observations near its perihelion on 9 September 2017, limiting its brightness to approximately 13.5 mag. By the end of October, ʻOumuamua had already faded to about apparent magnitude 23, and in mid-December 2017, it was too faint and fast moving to be studied by even the largest ground … A diagram showing the Konus drogue and module movements ... The orbital assembly of Mir began on 19 February 1986 with the launch of the Proton-K rocket. Four of the six modules which were later added (Kvant-2 in 1989, Kristall in 1990, Spektr in 1995 and Priroda in 1996) followed the same sequence to be added to the main Mir complex. Firstly, the module would be … An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s. 11.10.2021 · Spin Quantum Number. Let's think about an electron in an atom; first, imagine a spinning top. A top can spin clockwise or counter-clockwise. In the same way, an electron occupying an orbital ...
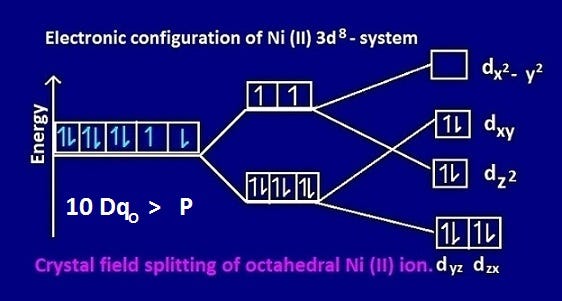
In this case, the d z 2 orbital drops even lower in energy, and the molecule has the following orbital splitting diagram. As a result of these distortions, there is a net lowering of energy (an increase in the ligand field stabilization energy) for complexes in which the metal has a d 7 , d 8 , or d 9 configurations, and thus electrons would ... 2. Molecular orbital theory: This is the best model to explain the bonding within the CO ligand as well as in metal carbonyl complexes. There are total three molecular diagrams for carbonyl ligand which were proposed from time to time. Though, all three molecular orbital (MO) diagrams are able to explain the nature of metal-4 Explanation: Electronic configuration of Nickel ( 28Ni) is. 1s2 2s2 2p6 3s2 3p6 4s2 3d8. After removal of two electrons from outermost shell the electron configuration of Ni2+ is. 1s2 2s2 2p6 3s2 3p6 4s0 3d8. Answer link. The orbital diagram for nickel is as follows. What is the orbital diagram for nickel. In all of the cases both up and down arrows are filled with the exception of the 3d shell where the last two are up. Were being asked to construct the orbital diagram for nifor that we first need to determine the electron configuration of ni.
We're being asked to construct the orbital diagram for Ni.For that, we first need to determine the electron configuration of Ni.. Recall that for a neutral element, Atomic number = # of protons = # of electrons. The atomic number of Ni is 28 and since it's a neutral element, this means Ni has 28 electrons.
01.11.2021 · Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of Rubidium …
To write the configuration for the Nickel ions, first we need to write the electron configuration for just Nickel (Ni). We first need to find the number of ...
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
Orbital Diagram. 1s ... Also in nickel-cadmium batteries; as a catalyst and for coins. Sources Chiefly found in pentlandite [(Ni,Fe)9S8] ore. The metal is produced by heating the ore in a blast furnace which replaces the sulfur with oxygen. The oxides are then treated with an acid that reacts with the iron not the nickel.
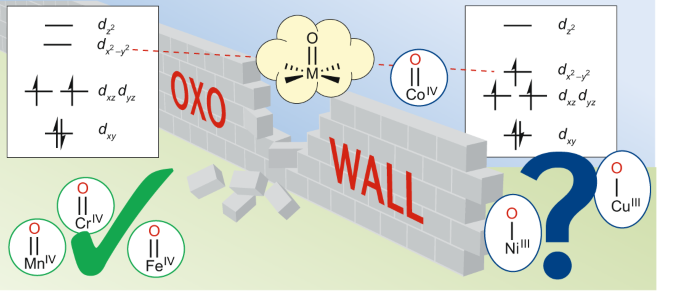
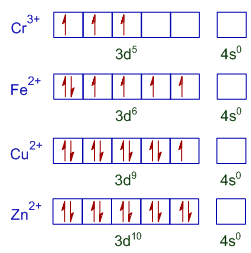
Draw both high spin and low spin d-orbital splitting diagrams for the following ions in an octahedral environment and determine the number of unpaired electrons in each case. a) Mn 2+ b) Co 2+ c) Ni 2+ d) Cu + e) Fe 3+ f) Cr 2+ g) Zn 2+ Answer
Orbital diagram. Nickel electron configuration ← Electronic configurations of elements . Ni (Nickel) is an element with position number 28 in the periodic table. Located in the ... Below is the electronic diagram of the Nickel atom Distribution of electrons over energy levels in the Ni atom 1-st level (K): 2 2-st level (L): 8 3-st level (M ...
What is the orbital diagram for nickel? Atomic Orbital Diagrams: Atomic orbital diagrams are also known as electron-in-a-box diagrams. These are simplified diagrams of how electrons are arranged ...
Binary compounds. Compound properties. Element reactions. Nickel atoms have 28 electrons and the shell structure is 2.8.16.2. The ground state electron configuration of ground state gaseous neutral nickel is [ Ar ]. 3d8. 4s2 and the term symbol is 3F4. Schematic electronic configuration of nickel. The Kossel shell structure of nickel.
The shape of the orbital ultimately determines the energy of the electrons. e. Electrons in the 2s orbital can penetrate the 1s orbital and be closer to the nucleus . e. Give the ground state electron configuration for I. ANSWER: a. [Kr]5s^24d^105p^5 b. [Kr]5s^25p^6 c. [Kr]4d^105p^6 d. [Kr]5s^25d^105p^6 e. [Kr]5s^24d^105p^6. a. Use the periodic table to determine the electron …
Answer and Explanation: 1. To help determine the electron configuration of nickel (Ni), we will visualize how the electrons are distributed in an Aufbau diagram below. Nickel has an atomic number ...
The orbital diagram for hydrogen can be represented in the following way. This notation uses a box to represent the orbital, the label for the orbital and an arrow to represent the electron. ... From iron through nickel ([Ar]4s 2 3d 6, [Ar]4s 2 3d 7, [Ar]4s 2 3d 8) the electrons spin-pair in the 3d sublevel. At copper another reversal occurs.
The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p64s2 3d8. In all of the cases, both up and down arrows are filled,with the exception of the 3d shell. Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first.
The diagram (not to scale) summarizes the energies of the orbitals up to the 4p level. Figure 1: Electronic energies orbitals. The oddity is the position of the 3d orbitals, which are shown at a slightly higher level than the 4s. This means that the 4s orbital which will fill first, followed by all the 3d orbitals and then the 4p orbitals.
Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund's Rule: Given sev...
Transcribed Image Textfrom this Question. Construct the orbital diagram for nickel 1 11 1 4p 1 11 14 1 3d 1 4s Answer Bank 1 1 1 3p 1 1 1L 3s 1 1 1L 2p 1 2s 1s Energy Construct the orbital diagram of the F ion Зр Answer Bank 3s 1 2p 2.s 1s Energy.
Orbital Diagram For Arsenic. Because the 4p section has 3 orbitals, but Arsenic ends with 4p3. It'll want to leave as few orbitals empty, so you have three arrows pointing up. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org!




/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)














0 Response to "37 orbital diagram for nickel"
Post a Comment