37 use the mo diagram provided below to answer the following questions: what is the bond order for n2?
In a diatomic molecule such as `O_2`, the bond order simply tells the number of bonds between the two atoms. The bond order can be interpreted from MO diagrams using the following formula: `"Bond Order" = 1/2 [("Bonding "e^-)-("Antibonding " e^-)]` The two formulas for bond order tell us the same information.
This problem has been solved! Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Use the MO diagram provided below to answer the following questions: • What is the bond order for C2?_ [Select] • Is C2 ...
No matter what kind of academic paper you need, it is simple and affordable to place your order with Achiever Essays. We have experienced writers in over 70+ disciplines for whom English is a native language and will easily prepare a paper according to your requirements. Order Now Free Inquiry. Calculate your paper price . Type of paper. Academic level. Deadline. Pages (550 …

Use the mo diagram provided below to answer the following questions: what is the bond order for n2?
Answer (1 of 2): Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons so first 2 electrons go in 1s sigma bond next 2 in 1s sigma anti bond orbital next 2 in 2s sigma bond orbital next 2 in 2s sigma...
Use the MO diagram provided below to answer the following questions: What is the bond order for N2? Is N2 paramagnetic or diamagnetic? What is the bond order for N2-? Is N2- paramagnetic or diamagnetic? What is the bond order for N2+? Is N2+ paramagnetic or diamagnetic? Which of the three has the longest bond? Which of the three has the ...
(a) Use the MO diagram from Figure 2.18 from the textbook: The electron configuration for O 2 - is 1σ g 2 1σ u 2 2σ g 2 2π u 4 2π g 3. This leaves 1 unpaired electron and gives a bond order of 1.5. (b) Use the same MO diagram as in (a), giving an electron configuration for O 2 + of: 1σ g 2 1σ u 2 2σ g 2 2π u 4 2π g 1.
Use the mo diagram provided below to answer the following questions: what is the bond order for n2?.
Draw the molecular orbital diagram for B 2. The number of unpaired electrons in the B 2 molecule is _____. (a) zero (b) 1 (c) 2 (d) 3 (e) 4 8. Which one of the following statements is false? (a) Valence bond theory and molecular orbital theory can be described as two different views of the same thing.
Question 8 predict the product for the following reaction
Transcribed image text: Use the MO diagram provided below to answer the following questions: • What is the bond order for N? [Select] • Is N2 paramagnetic or diamagnetic?__ (Select] netic? _ [ Select] • What is the bond order for N? (Select] • Is N2 paramagnetic or diamagnetic?__ (Select] • What is the bond order for Not?
Write the molecular orbital diagram of N2+ and calculate their bond order why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric. Explain
Re: Determining bond strength for N2, N2+, N2-, N2. Answer: For the purposes of this class, N2+ and N2- will be considered equal as they both have a bond order of 2.5. In advanced inorganic courses you will learn about group theory which is a tool to predict the energies of different molecular orbitals. By using a combination of group theory ...
A: MOT diagram for N2 is shown in next diagram. question_answer Q: It is discovered that it contains 7.759 % by mass of of the element oxygen (molar mass 16.00 g/mol...
For such an order you are expected to send a revision request and include all the instructions that should be followed by the writer. Also remember to state the exact time the writer should take to do your revision. We offer free revision as long as the client does not change the instructions that had been previously given. In case a client want to alter the instructions, revision can be done ...
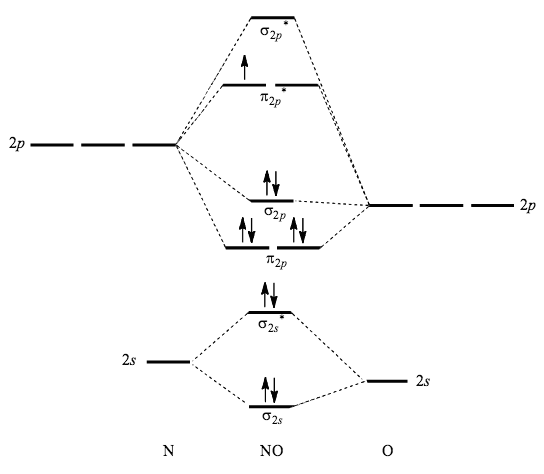
The MO diagram for "NO" is as follows (Miessler et al., Answer Key): (The original was this; I added the orbital depictions and symmetry labels. For further discussion on the orbital energy ordering being "N"_2-like, see here and comments.) Quick overview of what the labels correspond to what MOs: 1a_1 is the sigma_(2s) bonding MO. 2a_1 is the sigma_(2s)^"*" antibonding MO. 1b_1 is the pi_(2p ...
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves. Bond order for N2 is 3; bond order for N2- is and bond order for N2+ is I have not included pictures of the MO diagrams that show the orbital energies. N2+ has less bond energy.
4 Lecture 2 Pi bond (π): bonding molecular orbital -The bonding electron density lies above and below, or in front and in back of the bonding axis, with no electron directly on the bonding axis, since 2p orbitals do not have any electron density at the nucleus.
Solution for Use the MO diagram (below) to calculate the bond order for OF". Op Os menu. Products. Subjects. Business ... Use the MO diagram (below) to calculate the bond order for OF. Tp Op Os 1 3 Os 4 C 7 9. +/- х 100 + LO check_circle ... Find answers to questions asked by students like you.
If you're confident that a writer didn't follow your order details, ask for a refund. 25+ Subjects. From Literature to Law – we have MA and Ph.D. experts in almost any academic discipline, for any task. Any Paper. We can write, proofread, paraphrase, format, edit or rewrite your any paper, whether it’s a review or a term paper. High Quality. All the papers we deliver to clients are …
A plagiarism report from Turnitin can be attached to your order to ensure your paper's originality. Negotiable Price. Chat with your writer and come to an agreement about the most suitable price for you. No Hidden Charges. Every sweet feature you might think of is already included in the price, so there will be no unpleasant surprises at the checkout. 24/7/365 Support. You can …
Answer (1 of 6): The bond order of N2+ is 2.5 . * Total no. of electrons in N2+ : 13 * Its electronic configuration is : σ1s² σ*1s² σ2s² σ*2s² π 2py² [π2pz² σ2px1 ] * Bond order = 1/2[Nb-Na], where, Nb=no. of electrons in bonding molecular orbital and Na= no. of electrons in anti-bonding mole...
draw the molecular orbital diagram for B2. the number of electrons in the pi2p molecular orbital is. 1. ... what is the bond order for N2. 3. what is the bond order for O2 ... pi molecular orbital system extending above the plane of the,sigma system of the carbonate ion and an antibonding pi molecular orbital system extending below the plane of ...
2. Use the MO diagrams provided. 17. Refer to your MO diagrams. According to molecular orbital theory, what is the bond order for each of the following: a. C22- d. Li2 18. Refer to the MO Diagrams. According to molecular orbital theory, which of the following lists ranks the fluorine species in terms of increasing bond order? d. F2 < F22+ < F22 ...
The bond order tells us the stability of a bond: a higher bond order means the bond is more stable. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram.
The anion is phosphate, which is written as PO3−4. Step 3: Use the minimum number of cations and anions needed to make the sum of all charges in the formula equal to zero. The Li+ cation has a 1+ charge and the PO3−4 has a 3− charge. In order to balance the charges, three Li+ cations are needed for every PO3−4 anion.
Place the following in order of increasing X-A-X bond angle, where A represents the central atom and X represents the outer atoms in each molecule. ... The order of MO energies in B2, C2, and N2 (σ2p > π2p), is different from the order in O2, F2, and Ne2 This is due to _____. ... Use the molecular orbital diagram shown to determine which of ...
Questions. How do I order from Achiever Student? Place an order on our website is very easy and will only take a few minutes of your time. Click on the “order now” button to visit the order page. Fill the order form with your assignment instructions ensuring all important information about your order is included. Include your contact information so we can reach you if there are …
• The following diagram shows the molecular orbital energy level diagrams for the valence electrons in the homonuclear diatomic molecules C 2, N 2 and O 2. Complete the diagram by filling in the remaining valence electrons for each molecule and determining its bond order. Marks 6 Bond order: 2 3 2
Answer the following questions using your unit 3 notes. molecular orbital diagram practice Chapter 5 - Quantum Theory: electron configuration, orbital diagram, quantum numbers, Lewis diagram Chapter 9 - Chemical Names and Formulas Chapter 10 - Mole calculations, % composition, empirical and molecular formulae Orbital Filling Sequence And Energy Levels …
Answer (1 of 8): CN+ is a diatomic molecule, so to answer the question you need to start with two pieces of information: 1) the molecular orbital energy diagram for a diatomic molecule, and 2) the number of electrons in the molecule. CN+ has 12 electrons, 6 from C, 7from N, minus one for the posi...
To answer the questions, interpret the following Lewis diagram for Cho 1. Draw a box diagram to show the electron configuration of oxygen. ↿ . nitrogen B. (d) 1 triple bond between C and N, 1 N-H bond and 2 lone pairs of electrons on the C atom. mmm 3p 3s mma Is 1s22s22p33p6 Aluminum 1s22s22p63s23p1 3s Is Excited state electron configuration Identifr the element …
For such an order you are expected to send a revision request and include all the instructions that should be followed by the writer. Also remember to state the exact time the writer should take to do your revision. We offer free revision as long as the client does not change the instructions that had been previously given. In case a client want to alter the instructions, revision can be done ...
Bond order of B2 molecule. So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = .5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond.
Use the MO energy level diagram below to identify which one of the following statements is NOT correct. (Use only valence electrons) The superoxide has a stronger bond than the peroxide The superoxide has a shorter bond than the peroxide The bond order of the peroxide is 1 Oxygen, O2, has a longer bond than either of these oxides
Use the following Lewis structure for acetic acid to answer the following questions: ____ 36. The ideal value for the H-C H bond angle about atom 1 is: a. 90o b. 120o c. 109.5o d. 180o e. 60o ____ to decide whether it is polar or nonpolar.37. Draw the Lewis structure for SbF 3 a. polar b. nonpolar ____ 38. What does the following figure ...
Answer the following questions, which pertain to binary compounds. (a) In the box provided below, draw a complete Lewis electron-dot diagram for the IF 3 molecule. One point is earned for a correct Lewis diagram (can be done with dots or lines).
Use the figure provided to determine which of the following has bonds with the greatest bond-polarity. H2O Calculating the difference between the electronegativities for each element of each molecules, we find that H and O have the greatest difference, …
0 Response to "37 use the mo diagram provided below to answer the following questions: what is the bond order for n2?"
Post a Comment