38 lewis diagram for co2
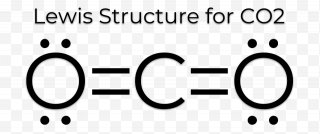
A step-by-step explanation of how to draw the CO2 Lewis Dot Structure (Carbon dioxide).For the CO2 structure use the periodic table to find the total number ... Carbon needs two double bonds, one to each of the two oxygens, to complete its octet. The atoms *share* electrons with each other because they are both non-m...
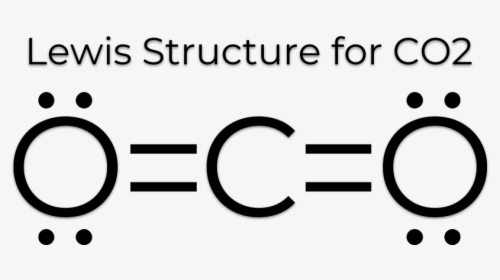
The Lewis Dot Structure for CO2. Created by MakeTheBrainHappy. This is the Lewis Dot Structure for CO2. You could alternatively also draw the structure by including two dots for every bond. That would mean that you would have a total of eight dots around the carbon, thereby filling its octet. The octets of both of the oxygen atoms are also ...

Lewis diagram for co2
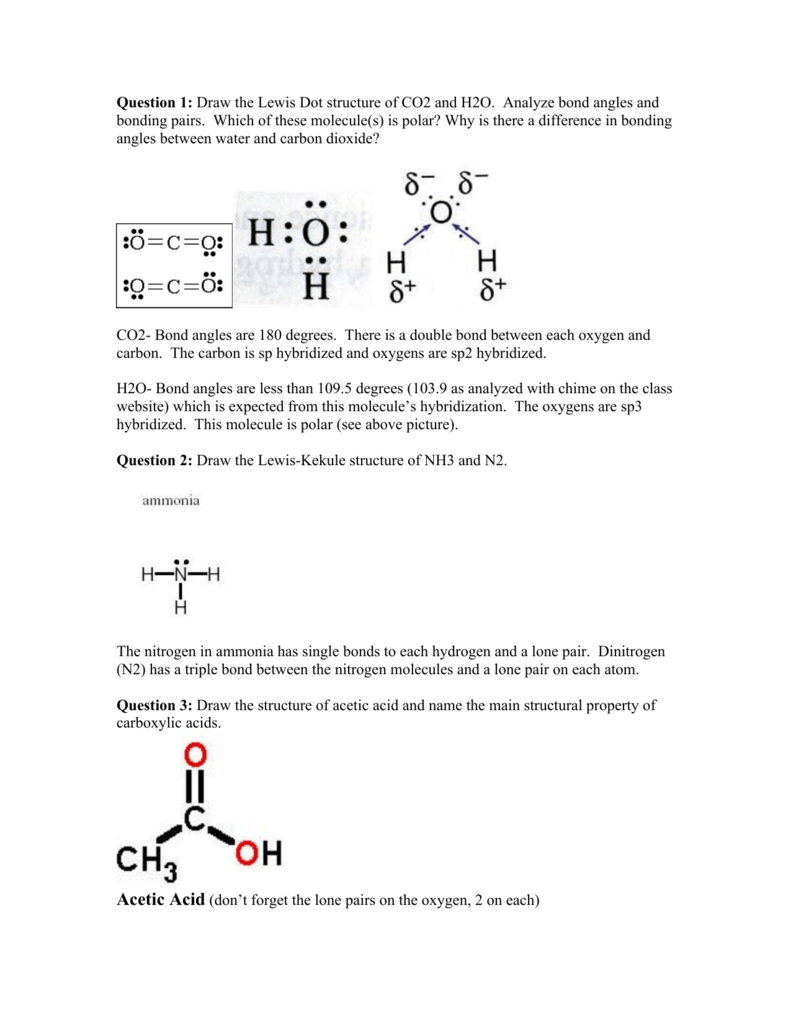
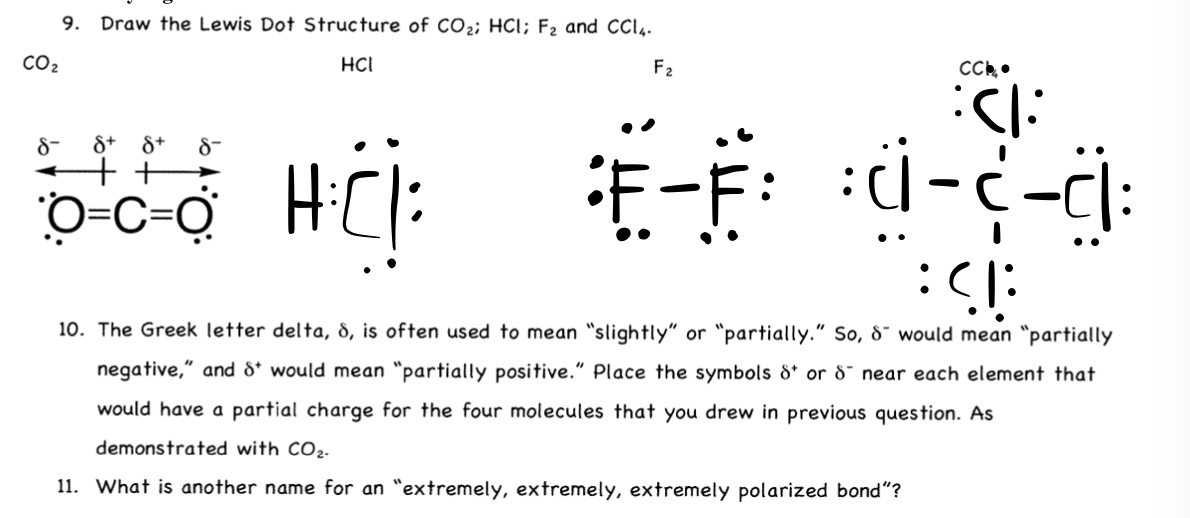
Carbon dioxide is not an acid itself, since it does not contain ions of hydrogen (H +). However, if CO 2 is dissolved in water it becomes carbonic acid which is a weak acid. 2. Explain CO2 Lewis structure in simple words. In the Carbon dioxide Lewis structure, the Carbon atom is in the central position as it is the least electronegative atom in ... In carbon dioxide, there are two oxygen atoms and one carbon atom, so, it means that carbon atom is the central atom and oxygen atom is attached to it. ... 4. Now ... Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure work on the octet rule, which means that all the atoms in the structure would have eight electrons in their valence shell except hydrogen.
Lewis diagram for co2. Carbon Dioxide is a Linear molecule, with AX2 geometry, a linear shape, and a 180 degree bond angle.Check me out: http://www.chemistnate.com What is the lewis structure for co2? Chemistry. 1 Answer anor277 May 22, 2018 #:ddotO=C=ddotO:# Explanation: Just to ... What is the lewis structure for hcn? How is vsepr used to classify molecules? What are the units used for the ideal gas law? How does Charle's law relate to breathing? ... Apr 18, 2015 · 1 answer1. www.shodor.org. 2. Two electrons (dots) make one bond (line). nonsibihighschool.org. A step-by-step explanation of how to draw the CO2 Lewis Dot Structure (Carbon dioxide).For the CO2 structure use the periodic table to find the total number ...
12+ Co2 Lewis Structure. To draw the lewis dot structure of co2, we have to find out the valence electrons of carbon and oxygen first.we express valence electrons as dots in lewis dot structure. Here are the steps that i follow when drawing a lewis structure. Chemists usually list this central atom first in the chemical formula (as in ccl4 and ... I quickly take you through how to draw the Lewis Structure of CO2 (Carbon DiOxide). I also go over hybridization, shape and bond angles. Draw the Lewis dot structure of CO2 molecule. Open in App Open_in_app ... expand. Solve any question of Chemical Bonding and Molecular Structure with:- ... In this article, we will discuss Carbon dioxide (CO2) lewis structure, molecular or electron geometry, hybridization, bond angle, polar or nonpolar, etc. At room temperature, carbon dioxide normally acts as gas but at high pressure, it turned into liquid. CO2 can be an environmental disaster due to its heat-absorbing properties.
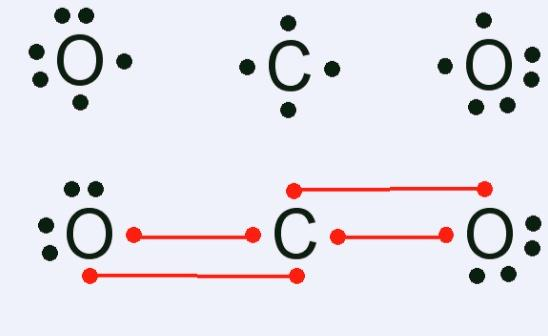
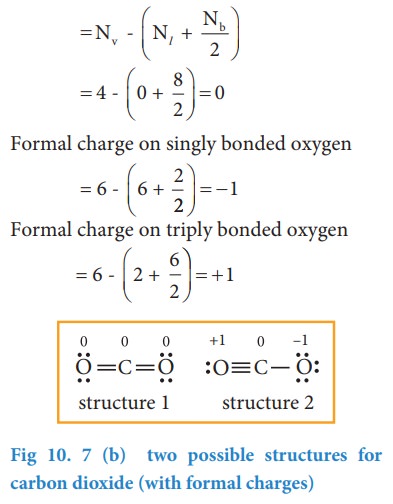
CO2 Lewis Structure. The lewis structure of CO2 can be with some simple steps, but before that, it is important to understand lewis structure properly. So lewis structure generally gives us an idea about the nature of bonding and octet fulfillment of the atoms. According to the octet rule, an atom attains stability by fulfilling its octet. Carbon dioxide (CO2) lewis structure has two double bonds around carbon atom. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO2 is linear. Steps of drawing the lewis structure of CO2 are explained. Let's go over the Lewis structure and find out how to interpret this representation of carbon dioxide. How To Read A Lewis Dot Structure. The Lewis dot structure is drawn with letters that represent the atoms of the element, and then a number of dots or dashes surrounding these letters. Dots can be used to represent the shared electrons ... 1 answerThe carbon atom and oxygen atoms have 4 and 6 valence electrons in them. This molecule has total electrons to be 16. The octet is complete by sharing the ...
30-gen-2021 - Hi Guys !In this video we are going to learn the Lewis dot structure of CO2 molecule. It is a chemical formula of Carbon Dioxide.
Lewis Diagram for Co2. the lewis dot structure for co2 makethebrainhappy learn what the lewis dot structure for co2 is in this article by makethebrainhappy co2 lewis structure how to draw the dot structure for co2 transcript ok this is dr b we re going to do the lewis structure for co2 carbon dioxide the periodic table carbon is in group 4 or 14 sometimes
Here is the Lewis structure of CO 2. CO2 Lewis structure. By following the above steps, you can draw the Lewis structure of any molecule. Here are some examples. Try to apply these five steps to the following examples and check whether your success or not. CO Lewis structure Step 01: calculation of total valence electrons of CO Step 02:
CO2 - C Mean Corbon And Corbon Have our Electron And O Means Oxygen. And Oxygen Have 6 Valence Electron And in Subscript oxygen mutiply with 2 So 6*2 = 12 And Add 4 Of Corbon. 4+6*2= 16. So The Co2 Lewis Structure is :..O=C=..O: . You Can Understand It With Image That Given Below. The Post. Draw Lewis Structure For Ccl4.
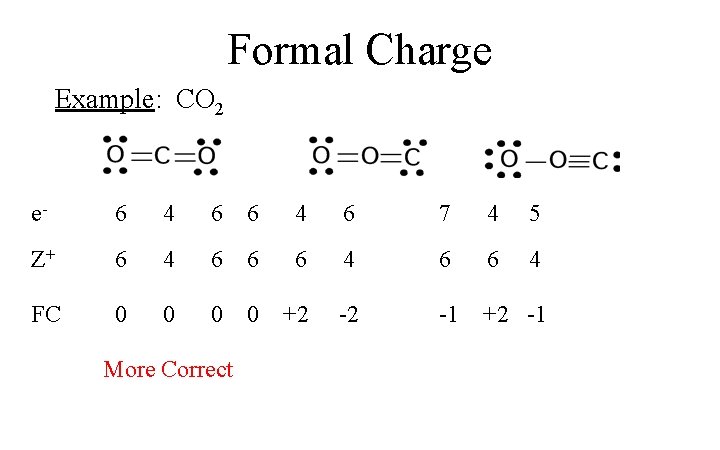
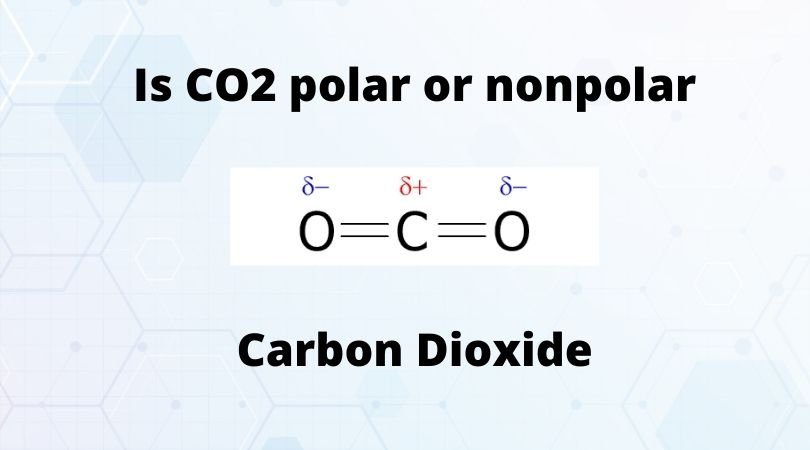
CO2 Lewis Properties. The CO 2 Lewis structure is symmetric. Generally, small symmetric molecules are nonpolar. CO 2 is a nonpolar substance, meaning it tends to be a gas. CO 2 has a rather low boiling point of around -80 ℃ or -100 ℉. It can be liquified and even frozen solid with special machinery to produce "dry ice.".
CO2 Lewis Structure. One needs to know the Lewis structure in order to understand the molecular geometry of any given molecule. This structure helps in knowing the arrangement of electrons in the molecules and the shape of the molecule. To know the lewis structure of CO2, one should first understand what precisely the Lewis structure is.
Well, we got underbrace(2xx6_"oxygen valence electrons"+4_"carbon valence electrons")_"16 electrons to be distributed over THREE centres" And the standard Lewis structure is... :ddotO=C=ddotO: ...which distributes 16 electrons, AS REQUIRED.... Because there are TWO regions of electron density situated around the central carbon, carbon dioxide is LINEAR with /_O-C-O=180^@...
Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure work on the octet rule, which means that all the atoms in the structure would have eight electrons in their valence shell except hydrogen.
In carbon dioxide, there are two oxygen atoms and one carbon atom, so, it means that carbon atom is the central atom and oxygen atom is attached to it. ... 4. Now ...
Carbon dioxide is not an acid itself, since it does not contain ions of hydrogen (H +). However, if CO 2 is dissolved in water it becomes carbonic acid which is a weak acid. 2. Explain CO2 Lewis structure in simple words. In the Carbon dioxide Lewis structure, the Carbon atom is in the central position as it is the least electronegative atom in ...
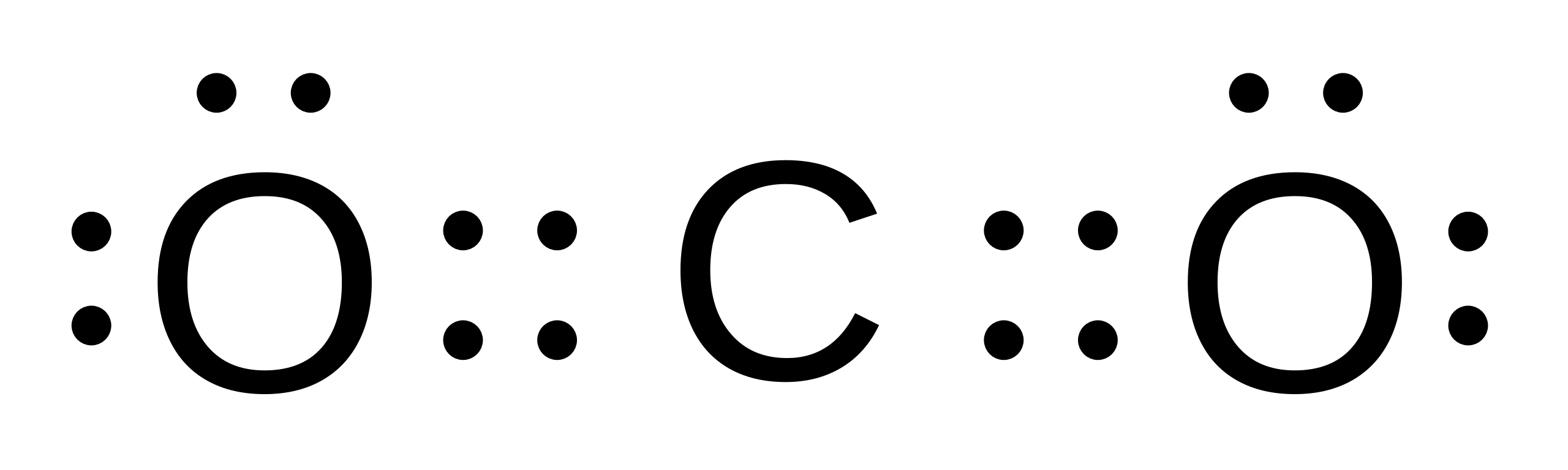














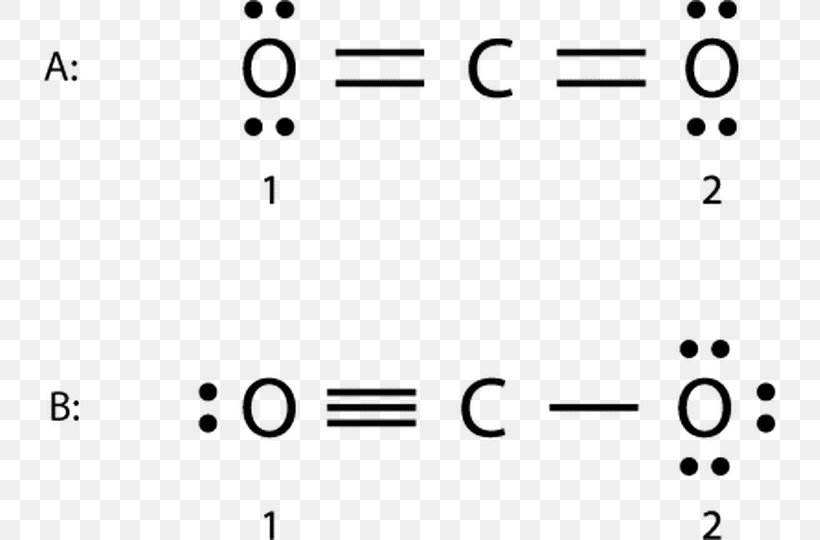








0 Response to "38 lewis diagram for co2"
Post a Comment