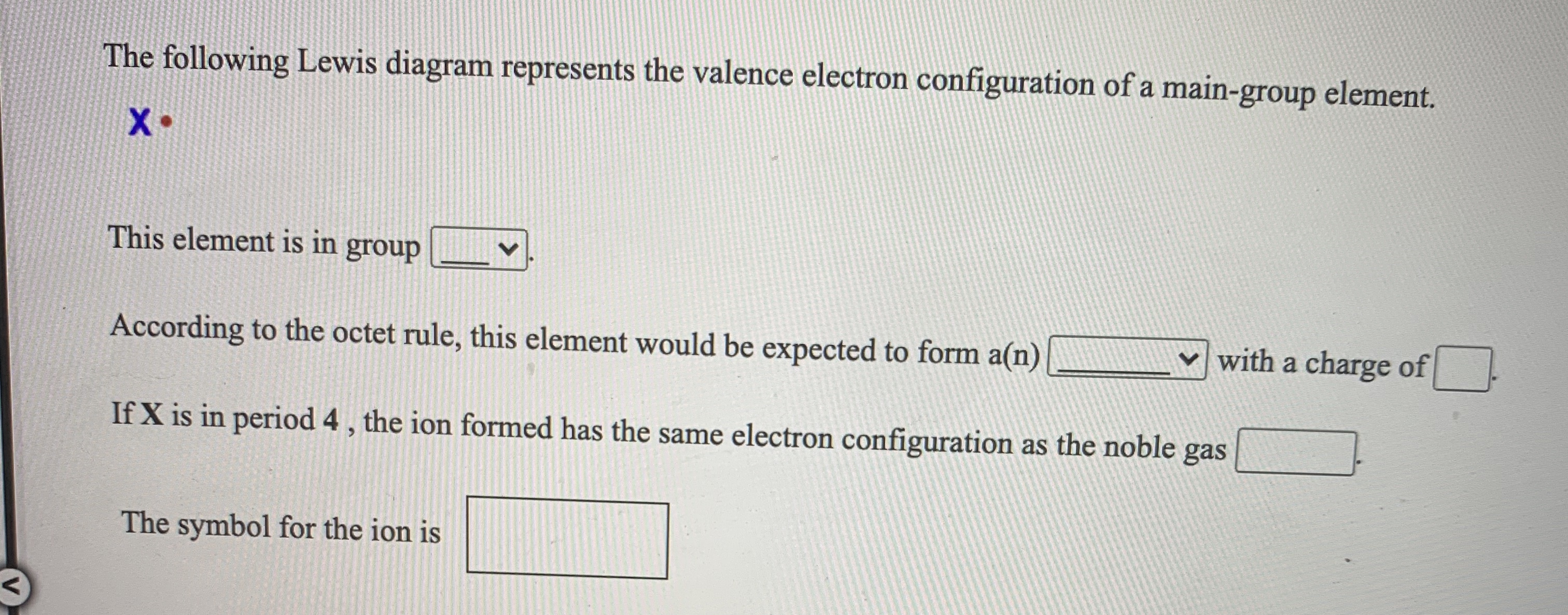
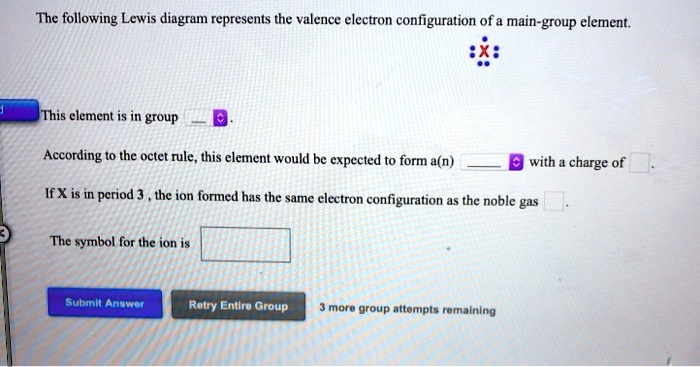
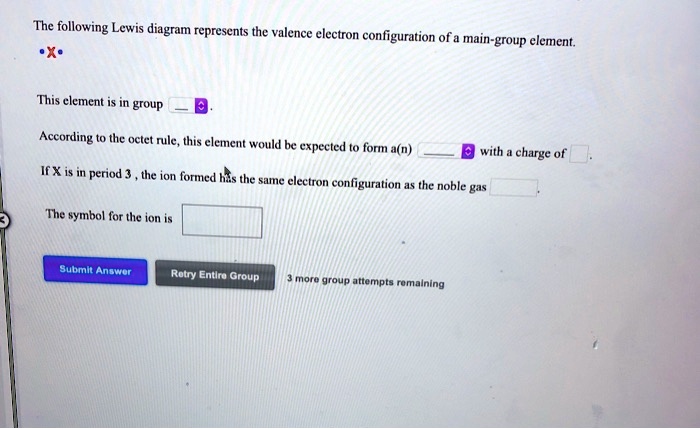
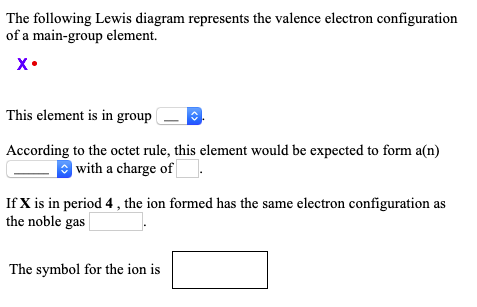
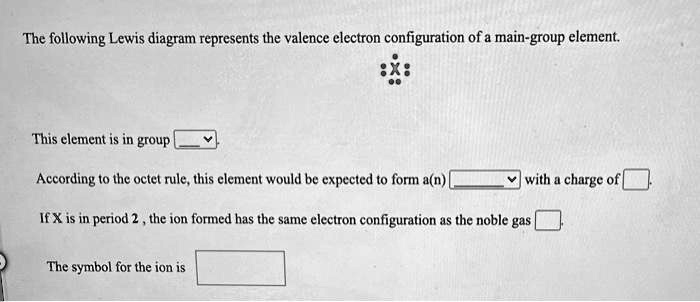
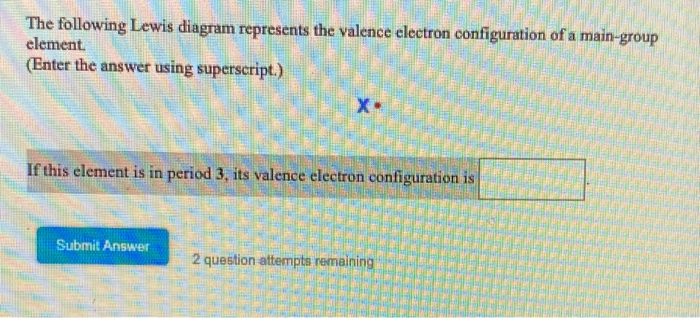
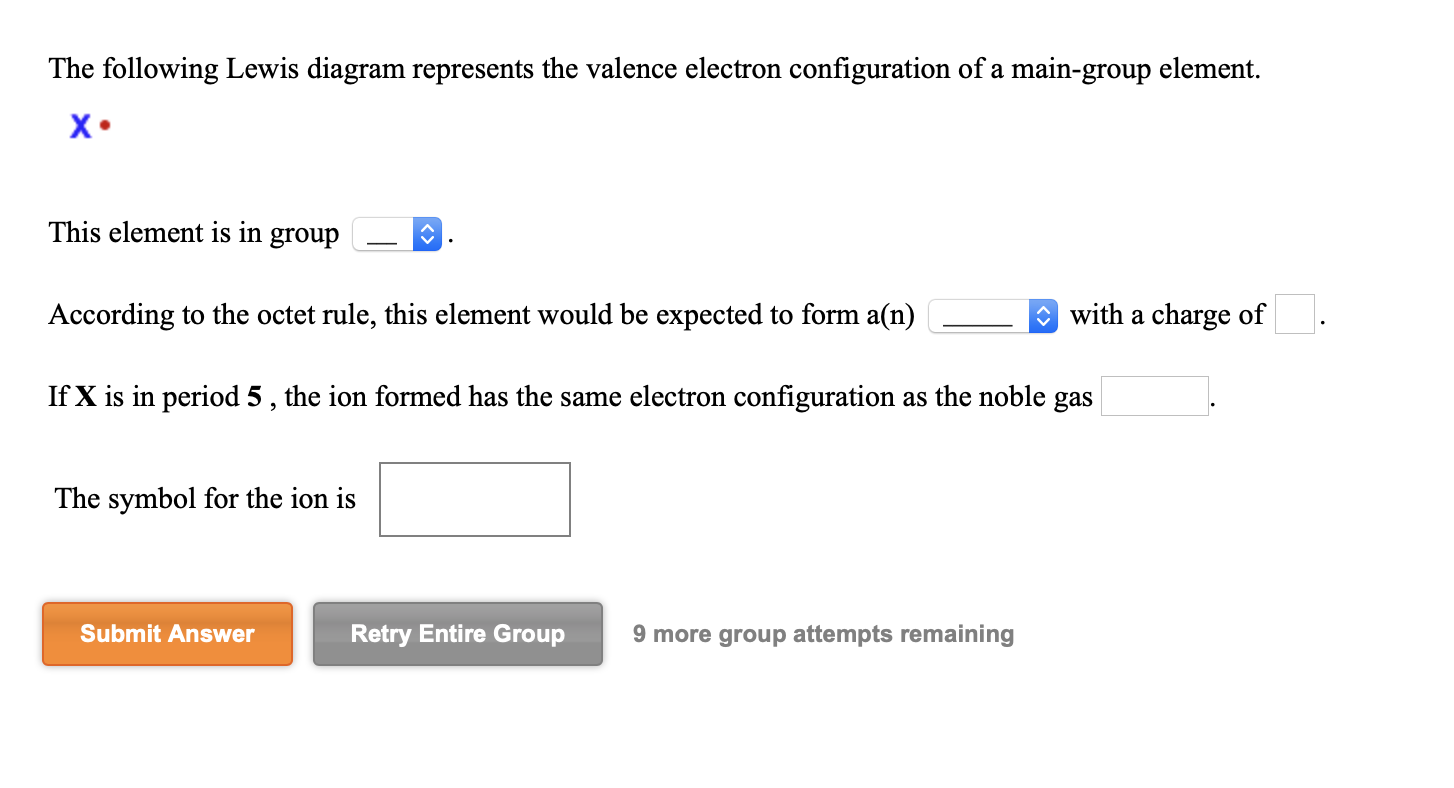
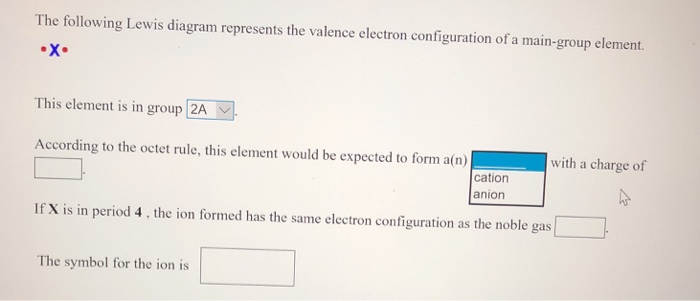
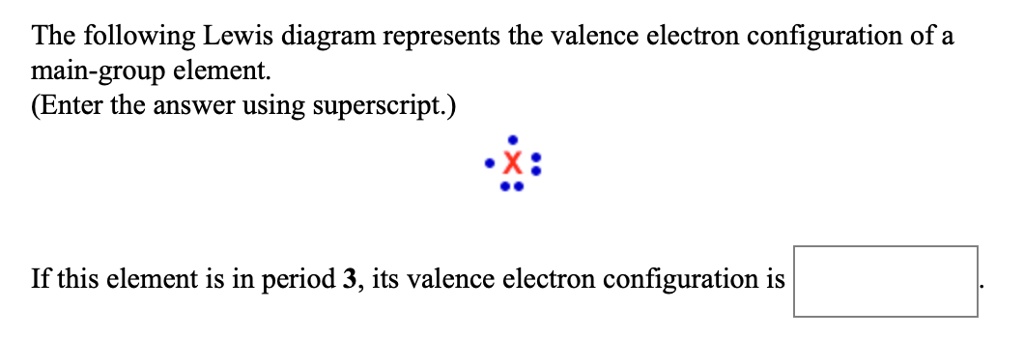
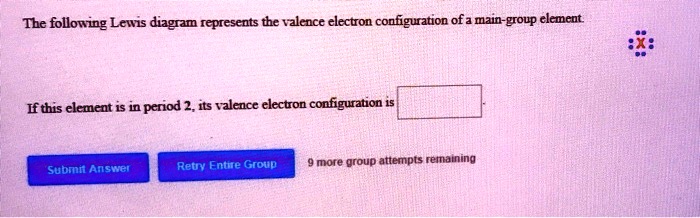
39 the following lewis diagram represents the valence electron configuration of a main-group element.
Sep 01, 2021 · The table below shows the electron arrangement for the first 20 elements in the Periodic Table along with their Element, Electrons diagram and configuration. Bohr model of the first 20 elements. Due to the technical limitations of my software, I will list the number of protons and neutrons of elements.
K + O2 ---> KO2 The Lewis structure of the superoxide anion contains two oxygens single-bonded, one with three lone pairs and - charged, the other with two and a half lone pairs. The central atom of a molecule is typically the atom with the highest electron valence of the atom with the lowest level of electronegativity.
HCN Lewis Structure, Molecular Geometry, Shape, and Polarity. Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. Represented by the chemical formula, HCN is one of those molecules that has an interesting Lewis structure. This liquid is used in electroplating, mining, and as a precursor for several compounds.
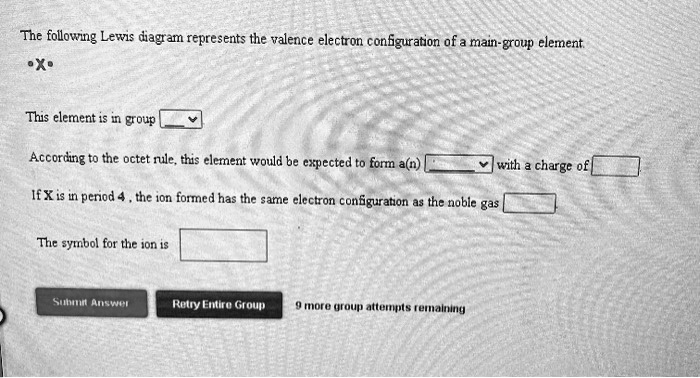
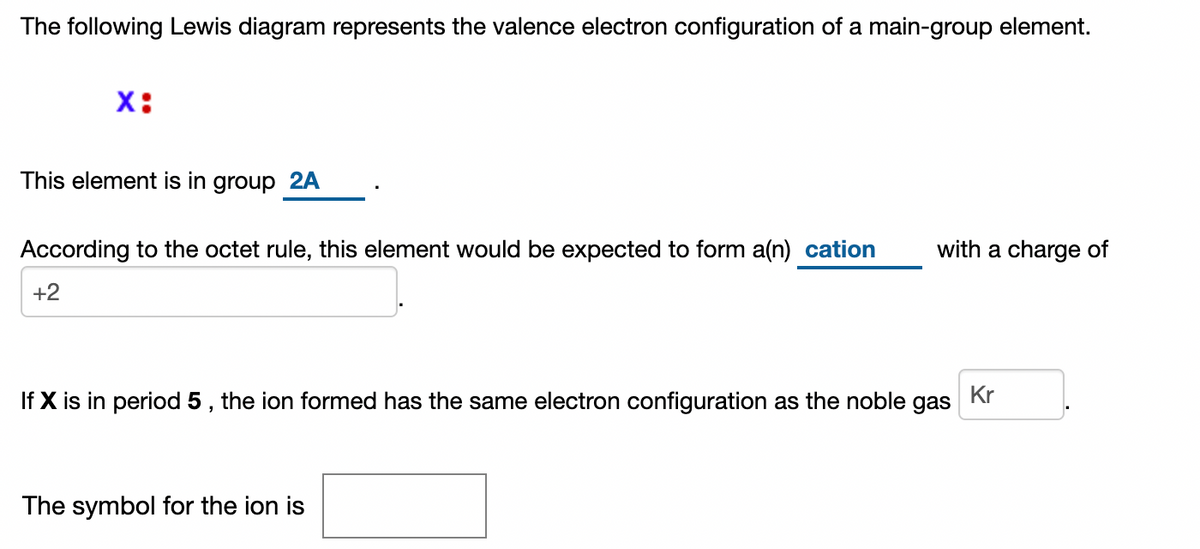
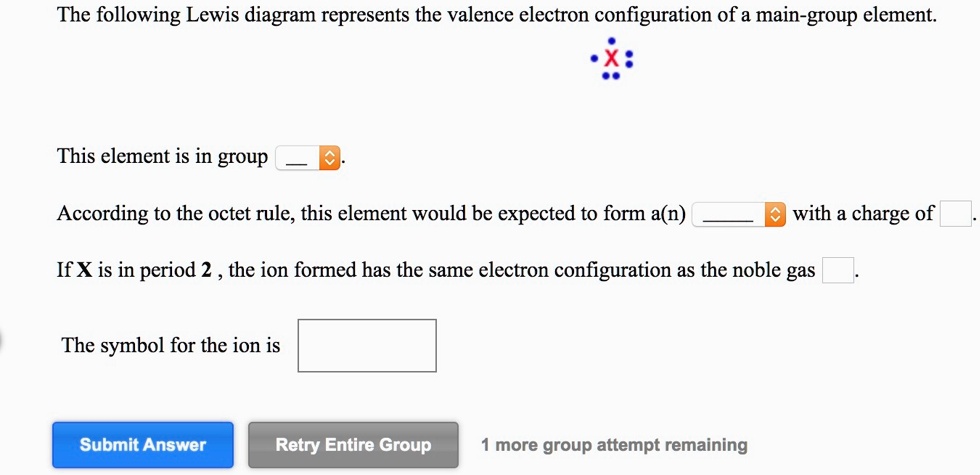
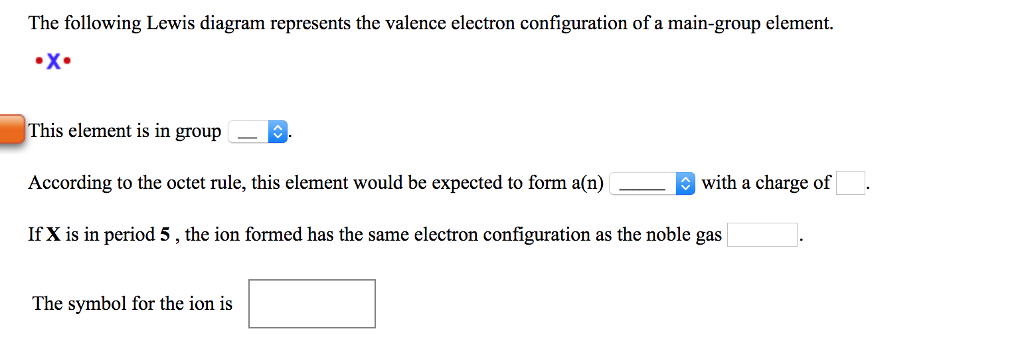
The following lewis diagram represents the valence electron configuration of a main-group element.
Mainly, the structure depicts the plan of the valence shell electrons of an element. An electron that is inserted in the outermost shell of one atom is known as a valence electron. To identify the variety of valence electrons, you can simply note down the Group variety of the aspect from the regular Table.
Reference: S. A valance that is gathered, measure 1 ½ times to double the width of your window. Feb 24, 2012 · Valence Electron and Electric Conductivity. 2 days ago · This calculator is used to find the bond polarity and tendency of electro-negativity in each element. Use a dot to represent an electron. Find more Chemistry widgets in ...
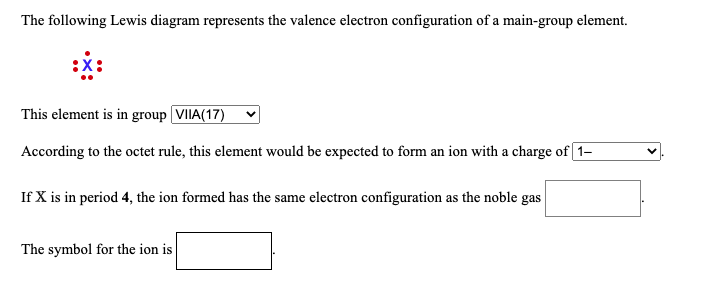
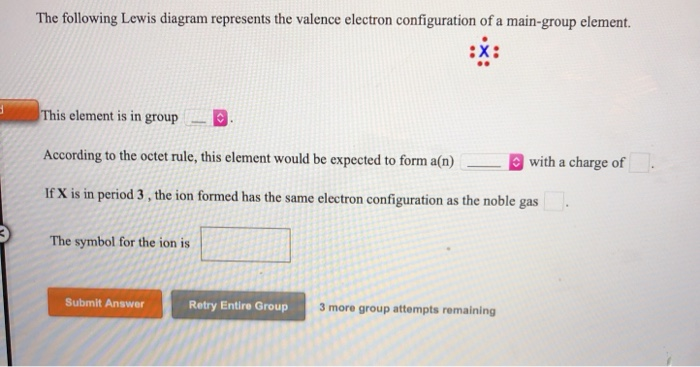
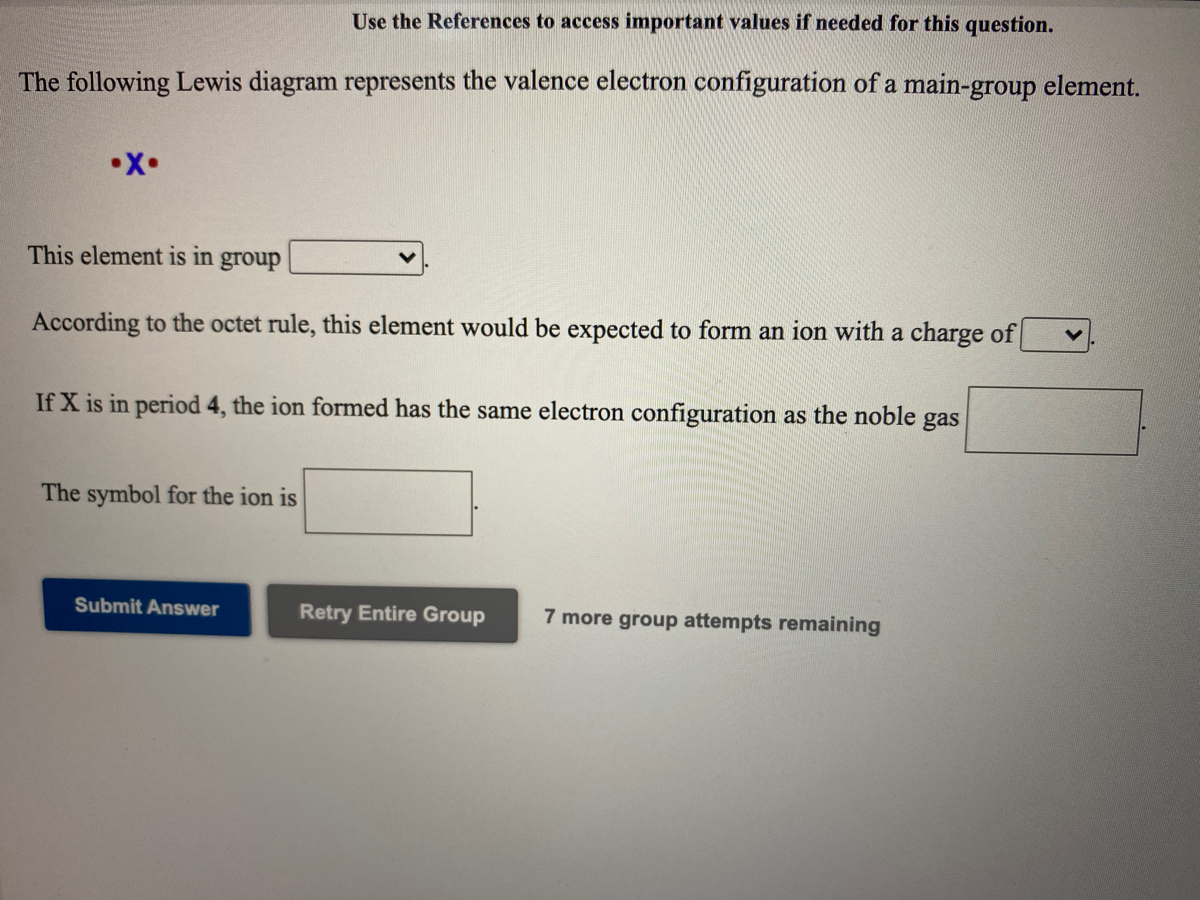
The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group According to the octet rule, this element would be expected to form a(n) wit
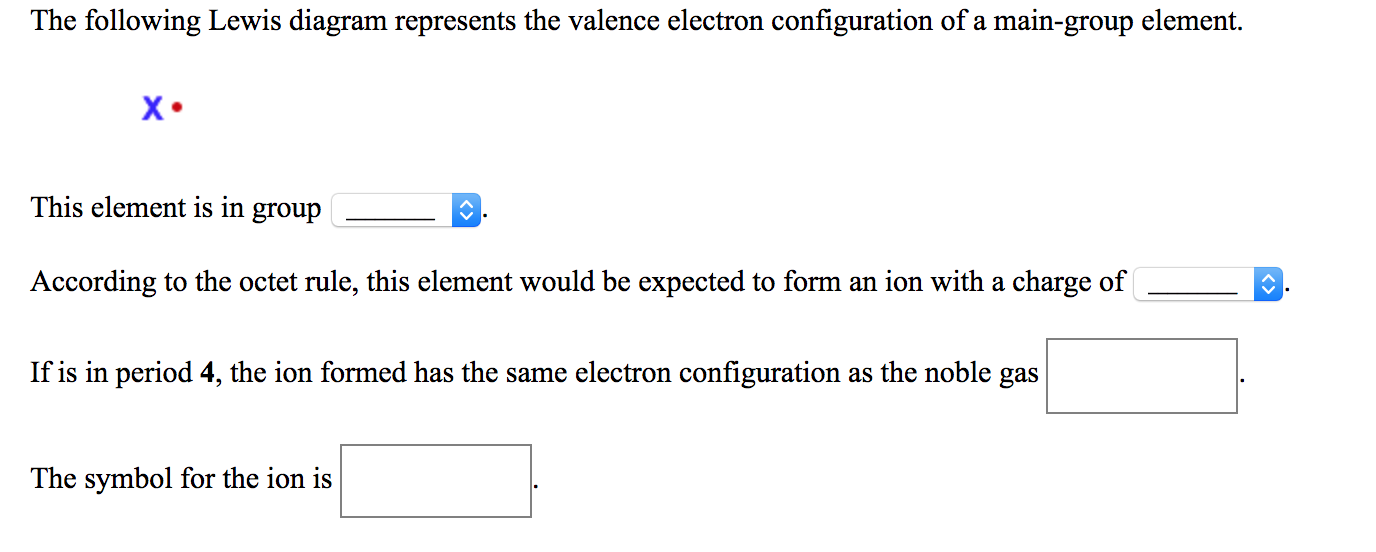
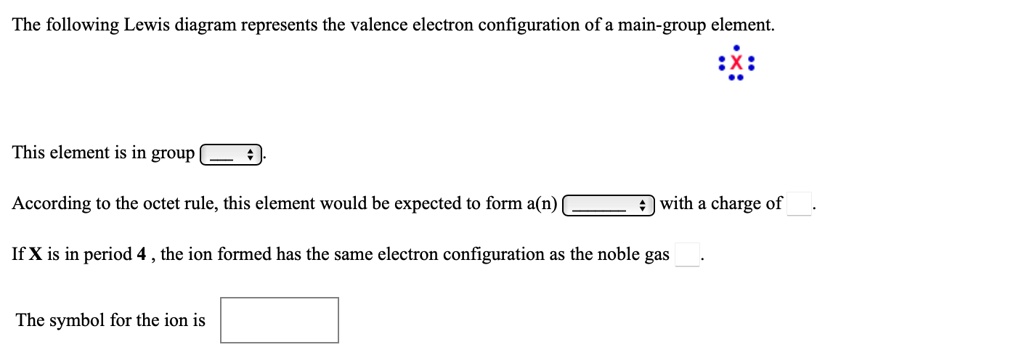
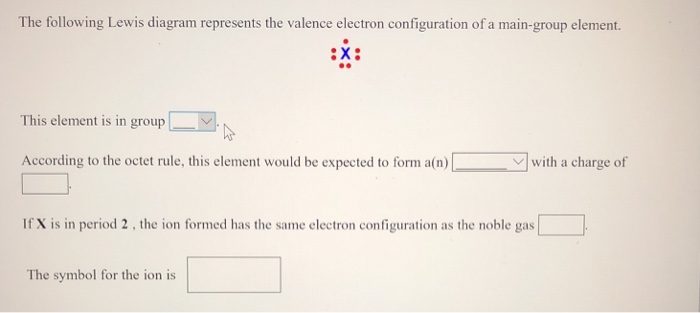
The following lewis diagram represents the valence electron configuration of a main-group element..
One dot . represents one valence electron. For the main group elements 1, the valence electrons are the electrons in the highest energy level (valence shell). Hydrogen, symbol H, has 1 valence electron, Lewis structure is H. Helium, symbol He, has 2 valence electrons, Lewis structure is He: With helium, the first energy level is full.
The main group number for an element can be found from its column on the periodic table. Lets draw it out as a simple diagram. An element in group 1A has 1 valence electron. Add together the valence electrons from each atom. The atomic number is how many protons and electrons the atom has.
Lewis structures also known as Lewis dot structures or electron dot structures are diagrams that represent the valence electrons of atoms within a molecule. Molecule proposed Lewis structure Is this a reasonable structure.
Group IA has one valence electron, so it loses it and becomes +1 charged. Groups IIA and IIIA lose two and three electrons, respectively, to become charged +2 and +3. Group IVA can go either way ...
Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s22s22p2. Now the main thing here is what electronic configuration actually means, so the solution/ answer is that in simple words, by knowing the electronic configuration of any ...
The Lewis structure of water suggests that this molecule has nonbonding pairs of valence electrons and can therefore act as a Lewis base. The electron configuration of Al 3 + ion suggests that this ion has empty 3 S, 3 p, and 3 d orbitals that can be used to hold pairs of nonbonding electrons donated by neighboring water molecules.
Lewis Structure of a compound is formed by arranging the atoms so that all the atoms obey the octet rule and formal charge is satisfied. This 2-D arrangement of atoms gives us a rough estimate of the bonds formed between different atoms. Octet Rule. Atoms of all main group elements want to have a fully filled valence configuration like that of ...
For atoms with 4 valence electrons it can go either way. 282016 82600 PM Company. Valence Electron Worksheet Name _____ Period _____ 1. Write orbital filling diagrams electron configurations and electron dot diagrams for the following elements. Valence Electrons Worksheet Chem. Law of Conservation of Matter and Electron Configuration Review 1.
An atom with one valence electron C. An atom with two valence electrons D. An atom with three valence electrons I think it is B. Arrange the elements in decreasing order of the number of valence electrons Br,S,Sb,Sr,F,Mg,I,Ca,Na,Kr,Xe,Bi,Ge,Ga,K. Valence Electrons Calculator; O2 Lewis Structure And Valence Electrons; O2 Valence Electron Structure
Electron Configuration for Magnesium Mg 1s2 2s2 2p6 3s2 Write the complete electron configuration for each of the following elements. In chemistry electron dot configuration has its own significance and this representation of valence electrons was invented by American chemist Gilbert Newton Lewis.
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
Steps to Draw the Lewis Structure of Cl2. 1. Count the total number of valence shell electrons on the molecule. This is done by adding the valence shell electrons of all the constituent atoms. Atom. Atomic Number. Group Number. Valence electrons according to group number. Electronic configuration (E.C.)
Outermost shell i.e. valence shell that participates in the chemical reaction and leads to the chemical combination. Lewis used specific notations to represent the valence electron which is known as the Lewis symbol. Group valence is either equal to no of dots in the Lewis symbol or 8 minus no of dots or valence electron.
A group represents elements with similar electron configuration, particularly the same number of valence electrons. Because of this, the elements of a group show similar chemical behavior.
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbital s, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. Oct 9, 2019 — The electronic configuration of a ground state chlorine is [Ne]3s23p5 (1s22s22p63s23p5).
Lewis structure diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion Lewis symbol symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion lone pair two (a pair of) valence electrons that are not used to form a covalent bond octet rule.
Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons …
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. Lewis symbols consist of an elemental symbol surrounded by one dot for each of its valence electrons: figure 1 shows Lewis symbols for elements of the third period of the periodic table.
For representative (main group) elements, valence electrons are those electrons _____. which occupy the outermost s and p orbitals. How many valence electrons does the representative (main group) element with the electron configuration 1s^2 2s^2 2p^6 3s^2 3p^5 possess?
The periodic table can be a powerful tool in predicting the electron configuration of an element. However, we do find exceptions to the order of filling of orbitals that are shown in Figure 3 or Figure 4.For instance, the electron configurations (shown in Figure 6) of the transition metals chromium (Cr; atomic number 24) and copper (Cu; atomic number 29), among others, are not …
A Lewis Structure or Electron Dot Structure is a very simplified representation of the valence shell electrons in a molecule. It denotes the way the valence electrons are arranged around the individual atoms in a molecule. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his 1916 article "The Atom and the Molecule ."
15 Lewis Dot Structure Examples. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. If there is not enough electrons to follow the octet rule, then the least electronegative atom is left short of electrons.
A core-abbreviated electron configuration (right) replaces the core electrons with the noble gas symbol whose configuration matches the core electron configuration of the other element. Similarly, the abbreviated configuration of lithium can be represented as [He]2 s 1 , where [He] represents the configuration of the helium atom, which is ...
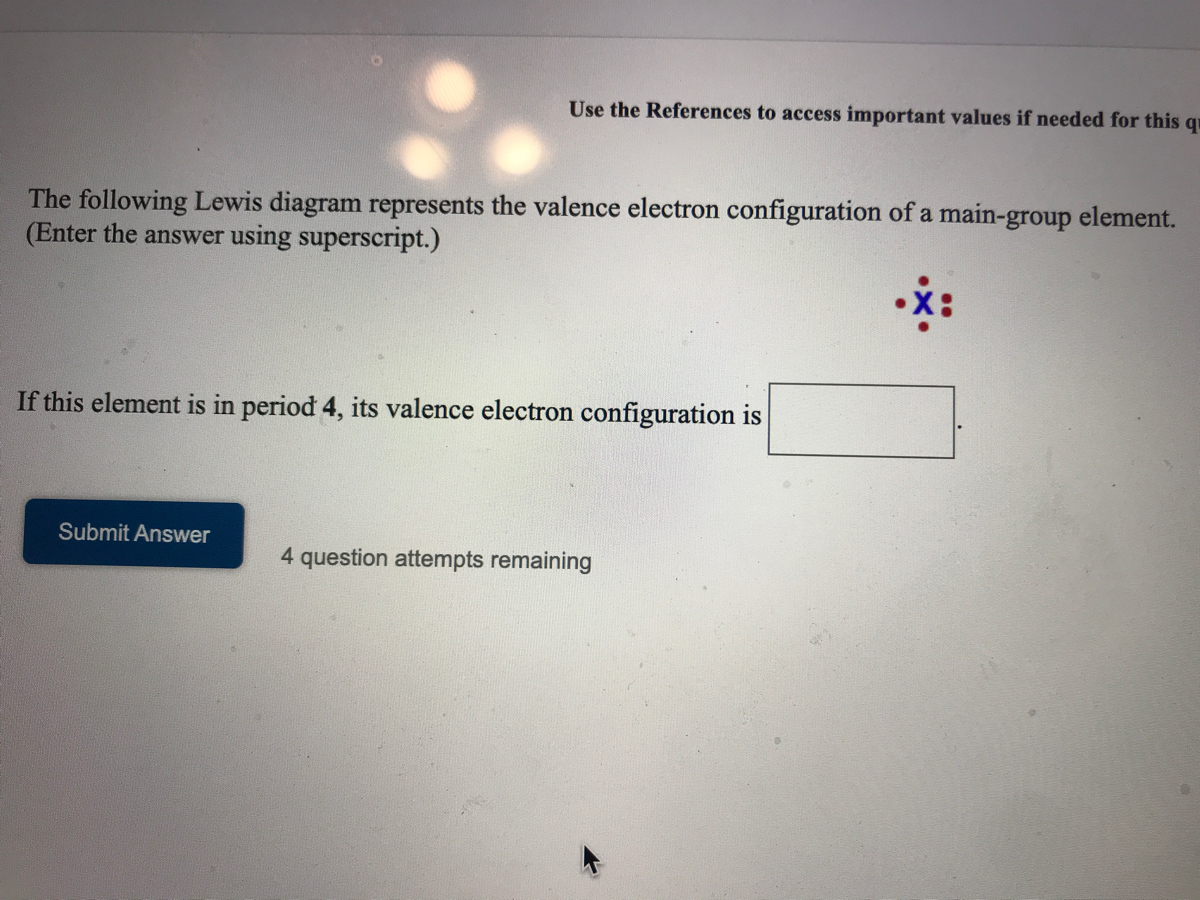
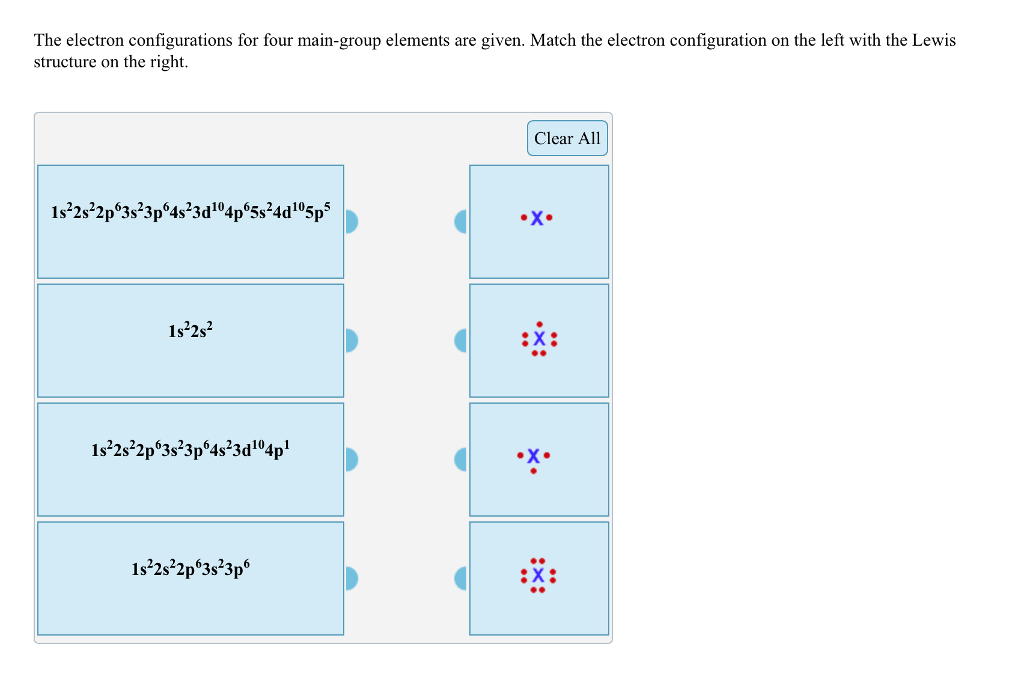
Those are the small number placed at the top after the letters. The electron configuration for Helium He is shown below. The following Lewis diagram represents the valence electron configuration of a main-group element. 1s22s22p63s23p64s23d104p6 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6. The symbol of bromine is Br.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. …. The total number of electrons does not change.
Feb 14, 2019 · Lewis Structures: The Basics. In a nutshell, a Lewis structure is a pictorial representation of the atomic structure and electron configuration of an atom or a compound.Single atoms are represented by their unique chemical symbol, electrons are represented as single dots, and shared pairs of electrons are represented by a single dash (−) …
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination …
All groups and messages ... ...
Electron Configuration Exceptions. The periodic table can be a powerful tool in predicting the electron configuration of an element. However, we do find exceptions to the order of filling of orbitals shown in Figure 3 and Figure 4.For instance, the electron configurations (shown in Figure 6) of the transition metals chromium (Cr; atomic number 24) and copper (Cu; atomic number …
Nov 12, 2018 · A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds.Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound.. Carbon tetrachloride (CCl 4) is a covalently bonded compound composed of a …
Lewis structures, here, comes into the picture where the valence electrons present in an atom are represented as dots. These structures are also known as electron dot diagrams. In Electron dot diagrams, an element is represented by its chemical symbol surrounded by dots representing the valence electrons.
Answer. Atomic Structure- 2 Q1. Draw the Lewis structure for the cyanide ion (CN-). The energy level an electron normally occupies is called its ground state.But it can move to a higher-energy, less-stable level, or shell, by absorbing energy. Therefore the Potassium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1.
To draw the Lewis dot structure, write the symbol for krypton, Kr, and then place two dots on the top, bottom, and two sides, for a total of eight electrons. Gallium is an element with atomic symbol Ga, atomic number 31, and atomic weight 69.7. Consider the following electron configuration. c.

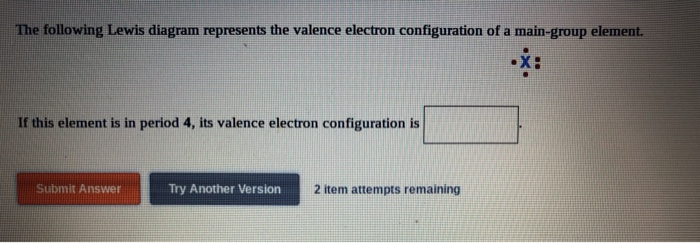
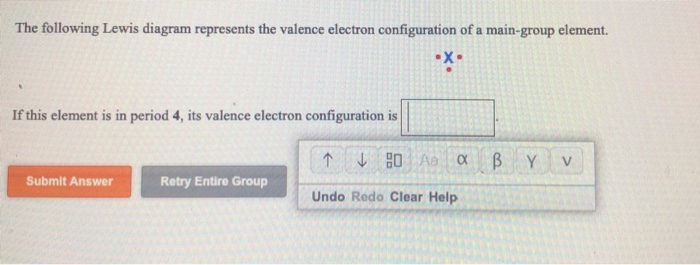
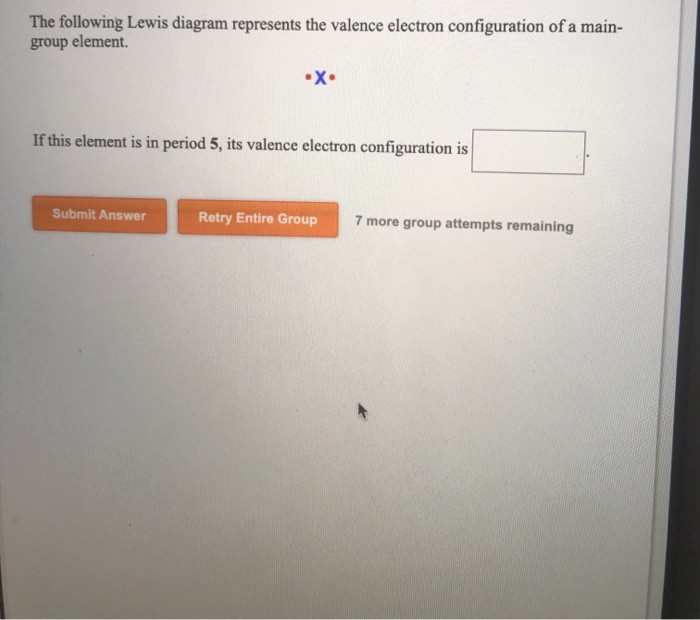
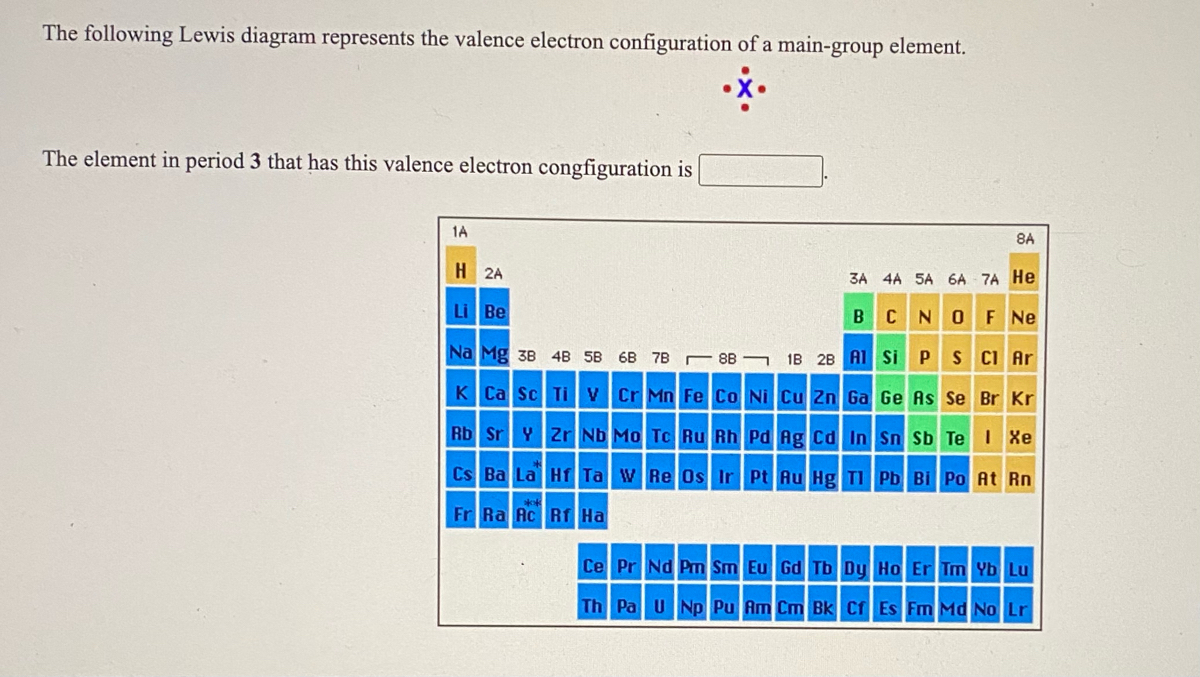
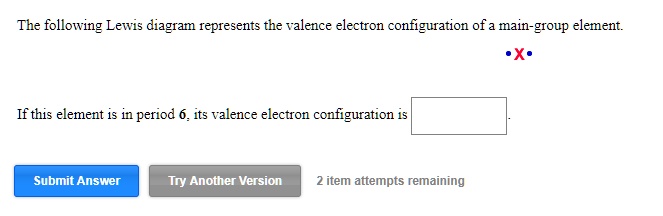
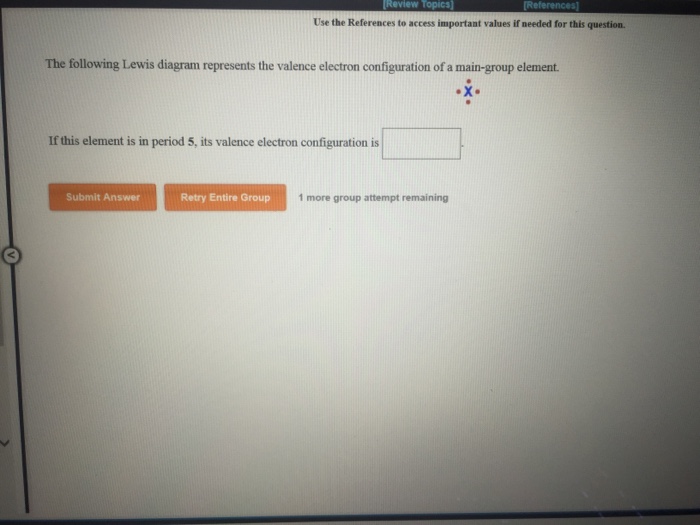
The following lewis diagram represents the valence electron configuration of a main-group element. if this element is in period 6, its valence electron configuration is:








0 Response to "39 the following lewis diagram represents the valence electron configuration of a main-group element."
Post a Comment