39 silicon lewis dot diagram
February 15, 2018 - Brainly.in is a part of the largest social network for studying in a group. We provide the best tools for mutual help with school subjects. Join us! AboutPressCopyrightContact usCreatorsAdvertiseDevelopersImpressumNetzDG TransparenzberichtNetzDG ComplaintsTermsPrivacyPolicy & SafetyHow YouTube worksTest new features · © 2021 Google LLC
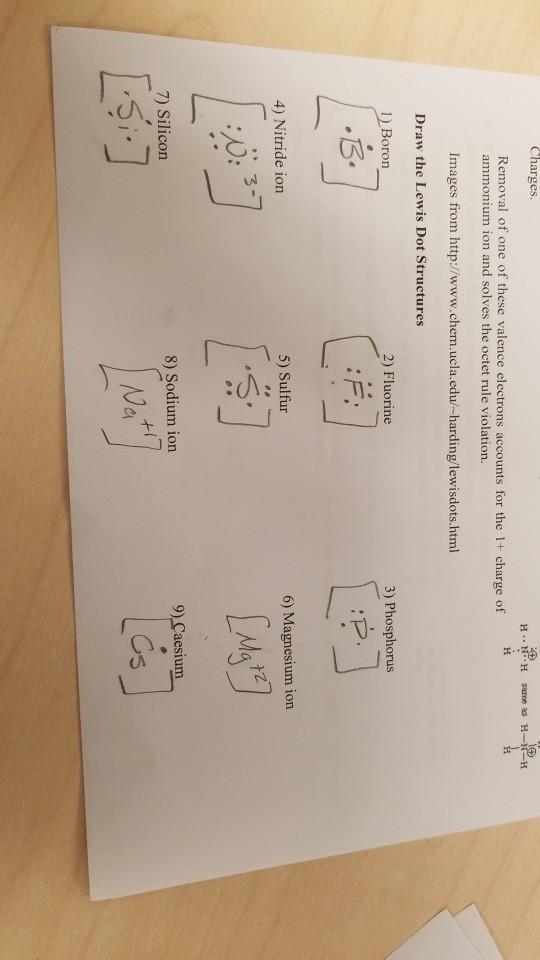
Write the electron dot (Lewis) diagrams for the following. 9. carbon silicon Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. Lewis structures extend the ...

Silicon lewis dot diagram
A Lewis electron dot diagramA representation of the valence electrons of an atom that uses dots around the symbol of the element. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. What is the Lewis Dot Structure Si? Silicon is in Group 14 (sometimes called Group IV or 4). Since it is in Group 4 it will have 4 valence electrons. When you draw the Lewis structure for Silicon you'll put four "dots" or valance electrons around the element symbol (Si). Hope this helped! 1.4K views View upvotes Related Answer Raida Innab Enjoy the videos and music you love, upload original content, and share it all with friends, family, and the world on YouTube.
Silicon lewis dot diagram. SiCl4 lewis’s structure is straightforward and very easy to draw. “Lewis diagram describes the chemical bonding of atoms within a molecule”. Lewis structure of SiCl4 contains 12 lone pairs on surrounding atoms and zero on the central atom. There are 4 bonding pairs present in the lewis structure of Silicon tetrachloride. SiO2 Lewis Structure (Step by Step Construction) In the SiO 2 lewis Structure, the overall ratio of silicon to the oxygen atom is 1:2.The Silicon oxygen bonds are strong and keep the atoms firmly in place. Following are the steps to construct the SiO 2 Lewis Structure.. Step-1: Count the valence electrons of atoms For the SiO 2 Lewis structure, we need to figure out the number of valence ... Silicon Oxide Lewis Dot Structure by LakeView Chemistry - February 15, 2013 Answer (1 of 2): Apparently there are different schools of thought on this (How to Draw Electron Dot Diagrams). That said, it seems that the one followed (at least in the Western US) has you start with the right hand side and the S orbital (only the outer valence electrons!): Si: (easier to do t...
A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. When constructing a Lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share electrons until they are surrounded by eight ... ! 56! Chapter5:Electron!Configuration,!LewisDot!Structure,!andMolecularShape !! Electron)configuration.)!! The!outermost!electrons!surrounding!an!atom(the!valence ... May 13, 2018 - Exercises explain why the first two dots in a lewis electron dot diagram are drawn on the same side of the atomic symbol. The lewis dot dia... A step-by-step explanation of how to draw the Lewis dot structure for Si (Silicon). I show you where Silicon is on the periodic table and how to determine ho...
Examples shown: Ammonia, Silicon Dioxide, Sulfur Hexafluoride Below is the image of the lewis dot structure of Silicon and Sulfur separately. Now let us study the steps involved to draw the Lewis structure of Silicon disulfide (SiS2): Step 1 : Note down the total number of valence electrons available to draw one molecule of silicon disulfide : It is 16 as 4 are coming from silicon atom and 6 are coming ... A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Silicon carbide is a yellow to green to bluish black iridescent crystals. The key is to understand the steps and practice. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
Construct the Lewis structure for the covalent compound silicon dioxide (SiO 2). Learn this topic by watching Lewis Dot Structures: Neutral Compounds Concept Videos All Chemistry Practice Problems Lewis Dot Structures: Neutral Compounds Practice Problems
Transcript: Hi, this is Dr. B. Let's do the SiO2 Lewis structure. On the periodic table, Si is in group 4, it has 4 valence electrons. Oxygen has 6, but we have two Oxygens, for a total of 16 valence electrons. We'll put the Si in the center and then the Oxygens on either side.
Laboratory Chemical Safety Summary (LCSS) Datasheet. Molecular Formula. S2Si. Synonyms. Silicon disulfide. 13759-10-9. Silicon sulfide (SiS2) bis (sulfanylidene)silane. UNII-35Y5PHW16K.
Get the best Lewis dot diagram for silicon, download apps, download spk for Windows, Android, Iphone.
Silicon tetraiodide is the chemical compound with the formula Si I 4. It is a tetrahedral molecule with Si-I bond lengths of 2.432 (5) Å. SiI 4 is a precursor to silicon amides of the formula Si (NR 2) 4 (R = alkyl). It has also been of interest in the manufacture and etching of silicon in microelectronics .
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
GHS Hazard Statements: H301 (100%): Toxic if swallowed [Danger Acute toxicity, oral]H311 (90.7%): Toxic in contact with skin [Danger Acute toxicity, dermal]H314 (100%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation]H317 (90.7%): May cause an allergic skin reaction [Warning Sensitization, Skin]H334 (90.7%): May cause allergy or asthma symptoms or breathing ...
What element has the same Lewis dot structure as silicon? No. What is the lewis dot diagram for carbon dioxide? Refer to the related link for an illustration of the Lewis dot diagram for carbon ...
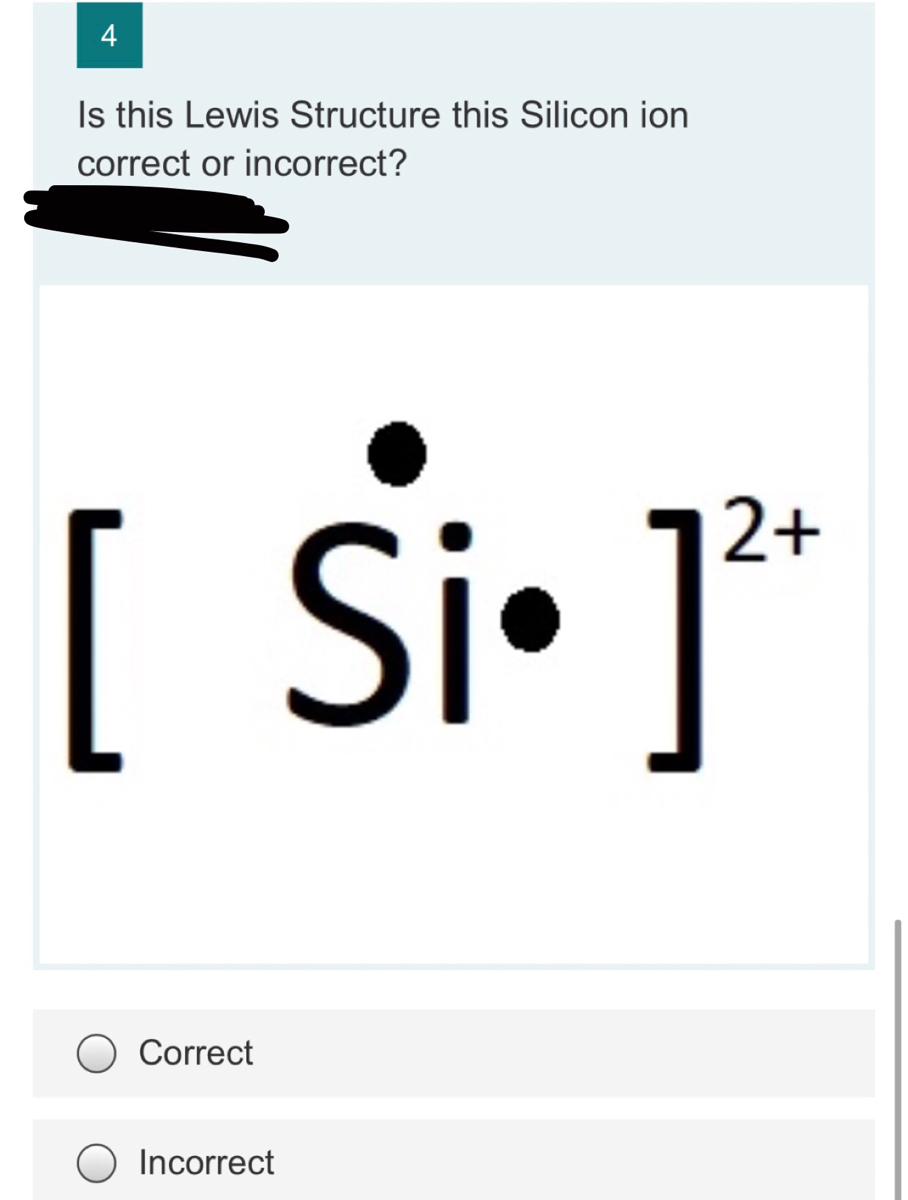
Silicon is in group 14 and period 3 of the periodic table and has four valence electrons in its Lewis structure. The four valence electrons means that silicon can bond in a way similar to carbon.
Write the electron dot (Lewis) diagrams for the following. 9. carbon silicon Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.
Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li
We will calculate the formal charge on the central atom of the SiO2 lewis diagram which is silicon. ⇒ Valence electron of Silicon = 4 ⇒ Bonding electrons = 8 ⇒ Non-bonding electrons (lone pairs electrons) = 0 Put these in given formal charge formula- ∴ (4 – 0 – 8/2) = 0 is the formal charge on the Silicon atom. Uses of Silicon dioxide.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
Which of the following elements has the same Lewis dot structure as silicon? Arsenic (As) Aluminum (Al) Gallium (Ga) Germanium (Ge) (Ge) How many hydrogen atoms are in H2SO,? 3. How many total atoms are in the glucose molecule C6h12o6. 18 6 24 3. Which compound below would most likely be a liquid or rgas at room temperature?
Dedicated To Excellence In Education · COVID-19 Positive Cases Dashboard
Is SiO2 Lewis dot structure? Silicon Dioxide (SiO2) Lewis Structure. The Lewis structure of SiO2 is identical to the Lewis structure of CO2. The only difference is that instead of carbon, silicon is used. One silicon atom is at the middle, with two oxygen atoms bound to it in a double bond.
Since there is no lone pair on the central atom of the SiO2 Lewis dot diagram, the bond angle is 180 degrees. This means there is no impact on the bond angle since there is no repulsion between the lone and bond pair. According to VSEPR theory, “The geometry around an atom with just two bonds and no unshared electrons is a straight line,”
A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure (Silicon Tetrahydride).For the SiH4 structure use the periodic table to find the total...
The lewis dot structure ignores the nucleus and all non-valence electrons, displaying only the valence electrons of an atom. If the atom is a noble-gas atom, two alternative procedures are possible. Either we can consider the atom to have zero valence electrons or we can regard the outermost filled shell as the valence shell.
Every chemistry student has to learn how to draw Lewis Dot Structures. The key is to understand the steps and practice. Lewis Structures are important to learn because they help us predict: the shape of a molecule. how the molecule might react with other molecules. the physical properties of the molecule (like boiling point, surface tension, etc.).
Lewis dot structure of SiO4− 4 S i O 4 4 − contains Si which is bonded to 4 oxygen atoms. Each oxygen atom is having a negative charge and the valence electrons of oxygen atoms are represented ...
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table.
June 25, 2017 - Silicon has four valence electrons. > Silicon is element 14 in the Periodic Table It has two electrons in its first shell, eight electrons in the second shell, and four electrons in the third shell. Since the electrons in the third shell are the outermost electrons, silicon has four valence ...
Answer (1 of 6): Silicon carbide (rarely: the mineral moissanite) is a refractory solid with a number of different allotropic covalent network structures. All of them have the atoms bound to four neighbors in a tetrahedral fashion with four covalent \sigma-bonds to the neighboring atom. One struc...
What is the Lewis diagram for f2? Drawing the Lewis Structure for F. 2 F2 is a reddish gas at room temperature. The F2 Lewis structure is similar to Br2, Cl2, and I2 since F, Br, Cl, and I are all in Group 7 and have 7 valence electrons. For the F2 Lewis structure there are a total of 14 valence electrons available.
A. Lewis electron dot diagram. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
Enjoy the videos and music you love, upload original content, and share it all with friends, family, and the world on YouTube.
What is the Lewis Dot Structure Si? Silicon is in Group 14 (sometimes called Group IV or 4). Since it is in Group 4 it will have 4 valence electrons. When you draw the Lewis structure for Silicon you'll put four "dots" or valance electrons around the element symbol (Si). Hope this helped! 1.4K views View upvotes Related Answer Raida Innab
A Lewis electron dot diagramA representation of the valence electrons of an atom that uses dots around the symbol of the element. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.













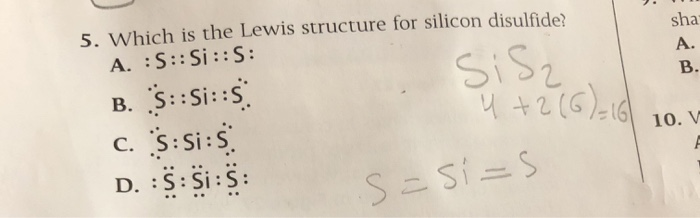



0 Response to "39 silicon lewis dot diagram"
Post a Comment