42 lewis dot diagram for lead
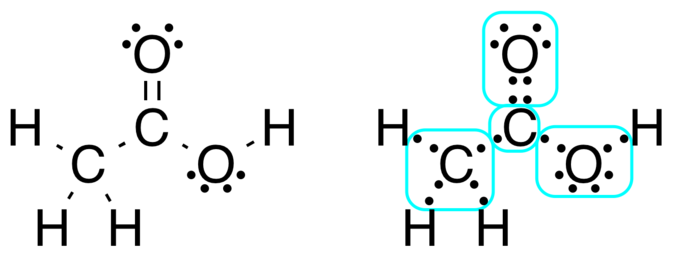
Chapter 20 / Lesson 6. 19K. Lewis dot structure is a diagram that represents the number of valence electrons of an element through dots around the element symbol. Learn about Lewis dots and ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
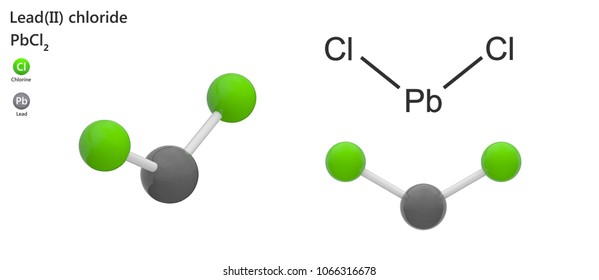
Skip to page content; Skip to site menu on this page. Periodic Table of Elements Element Lead - Pb. Comprehensive data on the chemical element Lead is provided on this page; including scores of properties, element names in many languages, most known nuclides of Lead.

Lewis dot diagram for lead
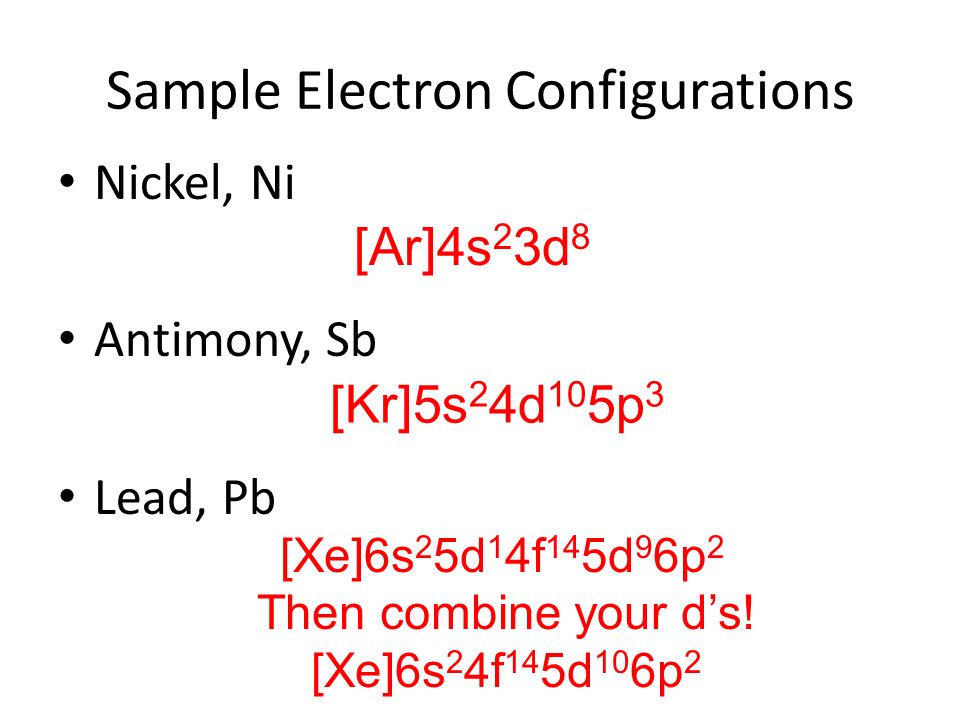
A step-by-step explanation of how to draw the Pb(OH)2 Lewis Dot Structure.For Pb(OH)2 we have an ionic compound and we need to take that into account when we... Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
Lewis dot diagram for lead. Nov 30, 2016 · Pb with four dots around it. Since Lead (Pb) has four valence electrons (Group 14), it gets four little spots to indicate the val e- around it. Note: Make sure to not pair them up( meaning put two dots beside each other) unless you have to. The Lewis Structure uses Hund's rule when placing the dots around the element. (Only for elements with 1-4 valence electrons do you have to follow the rule ... Lead (IV) Sulfide. Molecular Formula PbS. 2. Average mass 271.330 Da. Monoisotopic mass 271.920746 Da. ChemSpider ID 23253377. - Charge. This record has not been tagged. Names. Draw the Lewis dot structure for a lead atom. Question: ... Lewis dot structure is a diagram that represents the number of valence electrons of an element through dots around the element symbol ... Help. New Window. Lead (IV) dioxide occurs in nature as the mineral plattnerite (1). A plattnerite mineral sample from the Ojuela mine in Mexico assayed PbO2 99.6% and CuO 0.1% (2). Plattnerite occurs in weathered hydrothermal base-metal deposits, oxidized typically in arid climates (2).
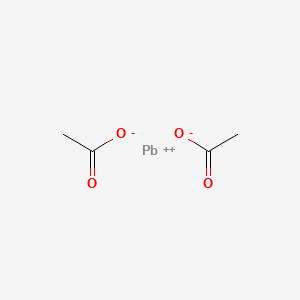
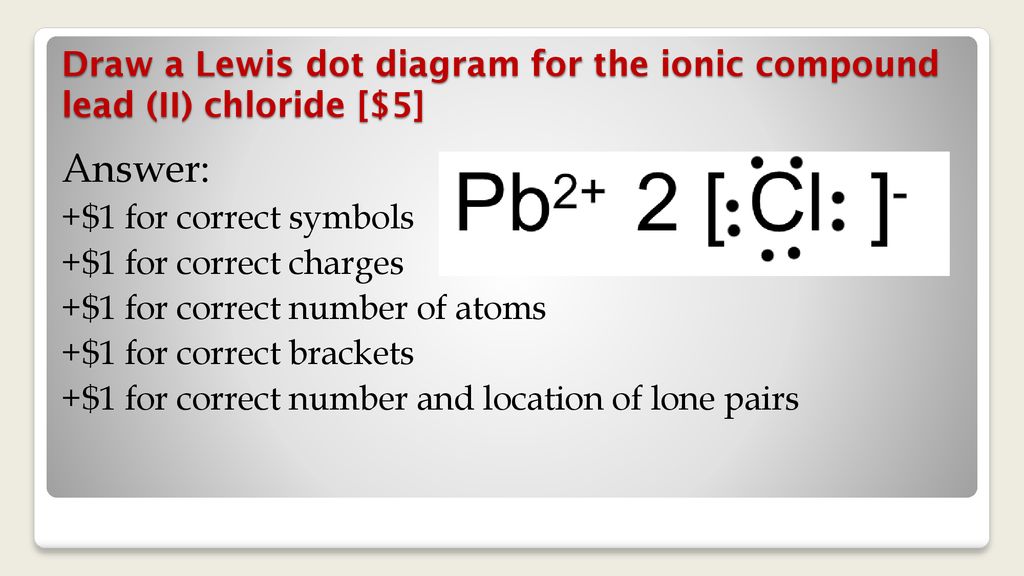
Lewis Structures for Polyatomic Ions. When writing dot structures for polyatomic ions, you must remember to add or subtract the amount of electrons represented by the charge. When writing polyatomic ions, you must include the structure inside brackets, [ ], with the charge outside the bracket via YouTube Capture The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. The Lewis structure of a positive ion (cation) is positioned adjacent to the Lewis structure of a negative ion (anion). If the charge on the positive ion is greater than the charge on the negative ion ... Lead(III) phosphate. Lead(IV) phosphate. Lead(II) phosphate. Tags: Question 22 . SURVEY . 30 seconds . Q. Which Lewis dot diagram accurately depicts the compound with the formula PCl 3? answer choices . Tags: Question 23 . SURVEY . 30 seconds . Q. Hydrogen chloride is a covalent compound. Which is a correct Lewis dot structure for HCl? answer ...
Mr. Andersen shows you how to draw Lewis Dot Diagrams for atoms and simple molecules. Sep 14, 2011 · The Lewis structure for lead has 2 electrons. ~*~ The above answer is actually completely incorrect. the very first shell does not have 8 valence electrons it has two. Because of this there are 4 ... ospheric chemical reactions between ozone and free radical species, such as nitric monoxide (NO) and nitrous oxide (NO2), can lead to the destruction of ozone, causing a depletion of the ozone layer and putting life on Earth at increased risk of exposure to UV light. Draw the Lewis dot structures for the free radicals of NO and NO2 NO, NO. Lewis dot diagrams give us a static picture of what the molecule or ion might look like. So how do we deal with situations were a static model does not tell us the entire story. We assume that we can represent each different configuration by a Lewis dot structure and in some cases, all these different diagrams add to our overall understanding ...
Lewis structures, also known as electron dot structures, are named after Gilbert N. Lewis, who described them in a 1916 article titled, "The Atom and the Molecule." Lewis structures depict the bonds between atoms of a molecule, as well as any unbonded electron pairs. You can draw a Lewis dot structure for any covalent molecule or coordination ...
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule.
Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral.
In the Lewis structures listed here, M and X represent various elements in the third period of the periodic table. Write the formula of each compound using the chemical symbols of each element: (a) (b) (c) (d) Write the Lewis structure for the diatomic molecule P 2, an unstable form of phosphorus found in high-temperature phosphorus vapor.
A step-by-step explanation of how to draw the Pb(NO3)2 Lewis Dot Structure.For Pb(NO3)2 we have an ionic compound and we need to take that into account when ...
The lewis dot structure ignores the nucleus and all non-valence electrons, displaying only the valence electrons of an atom. If the atom is a noble-gas atom, two alternative procedures are possible. Either we can consider the atom to have zero valence electrons or we can regard the outermost filled shell as the valence shell.
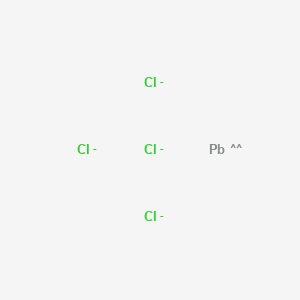
The Lewis structure shows the calcium with no dots (electrons), and the chlorine ions with a complete octet. Notice the placement of the charge notation on the ions. 3. The Ca and Cls are near each other, but the two dots (electrons) from each Cl should not be interpreted as a covalent bond. The final Lewis dot structure is as follows: 4.
Answer (1 of 2): To draw lewis dot structures, you first must know the number of valence electrons the atom has. By writing Fe in a noble gas configuration, you get [Ar] 3d6 4s2. By looking at this, since iron is a transition metal, you can see that iron technically has 8 valence electrons, but w...
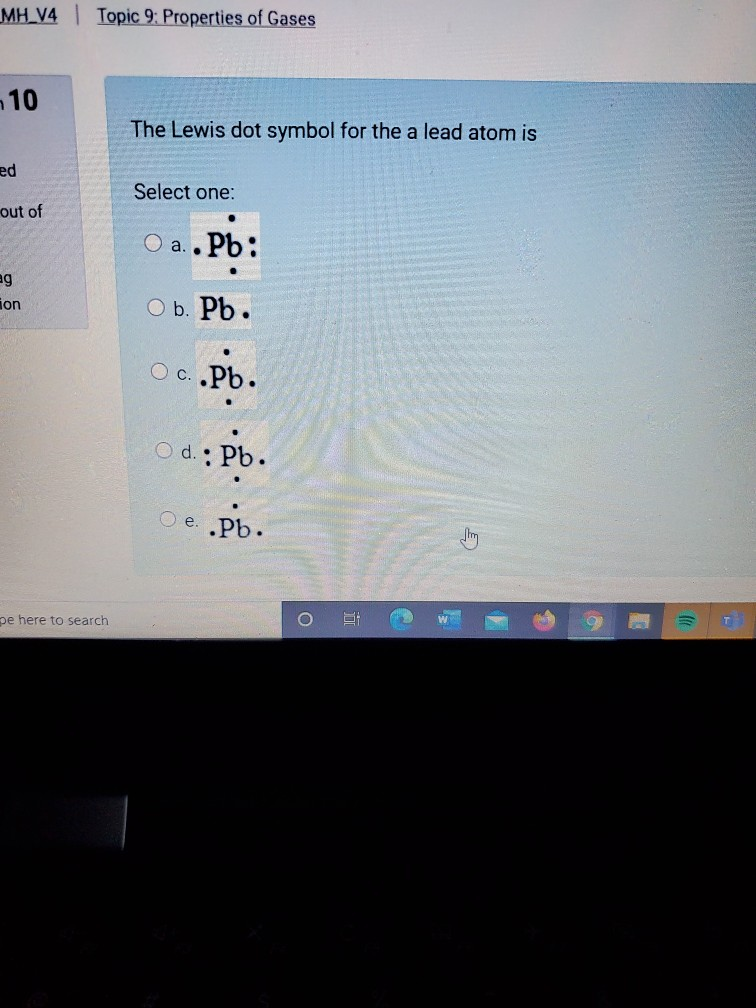
The Lewis dot symbol for the a lead atom is. ... The Lewis structure for CS₂ is: What is a useful guideline for the application of formal charges in neutral molecules? A Lewis structure in which there are no formal charges is preferred. In the Lewis structure of the iodate ion, IO₃⁻, that satisfies the octet rule, the formal charge on the ...
Lewis structures go by many names, including Lewis electron dot structures, Lewis dot diagrams, and electron dot structures. All these names refer to the same sort of diagram, which is intended to show the locations of bonds and electron pairs.
Lewis dot structures (or just Lewis structures) were developed around 1920 by pioneering chemist Gilbert Lewis, as a way of picturing chemical bonding in molecules.. We draw Lewis structures to . Discover the bonding arrangement of atoms,; Discover whether there is any degeneracy of bonding (more on that later),; Figure out whether a given group of atoms might even bond together to form a ...
2021-11-06. Create. 2005-03-27. Lead iodide appears as a yellow crystalline solid. Insoluble in water and denser than water. Primary hazard is threat to the environment. Immediate steps should be taken to limit spread to the environment. Used in printing and photography, to seed clouds and other uses. CAMEO Chemicals.
A Lewis dot diagram for arsenic (As) would have how many dots around the chemical symbol? 5. Element X has an electron configuration that ends with 5p4. How many electrons would it like to gain to achieve stability? 2. Element X has an electron configuration that ends with 3p6.
Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
A step-by-step explanation of how to draw the Pb(OH)2 Lewis Dot Structure.For Pb(OH)2 we have an ionic compound and we need to take that into account when we...









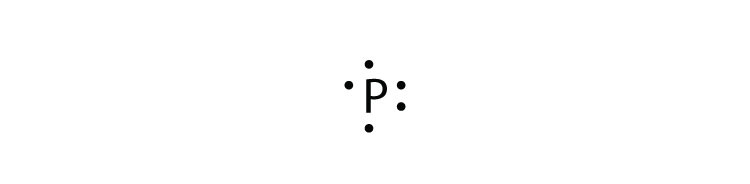









0 Response to "42 lewis dot diagram for lead"
Post a Comment