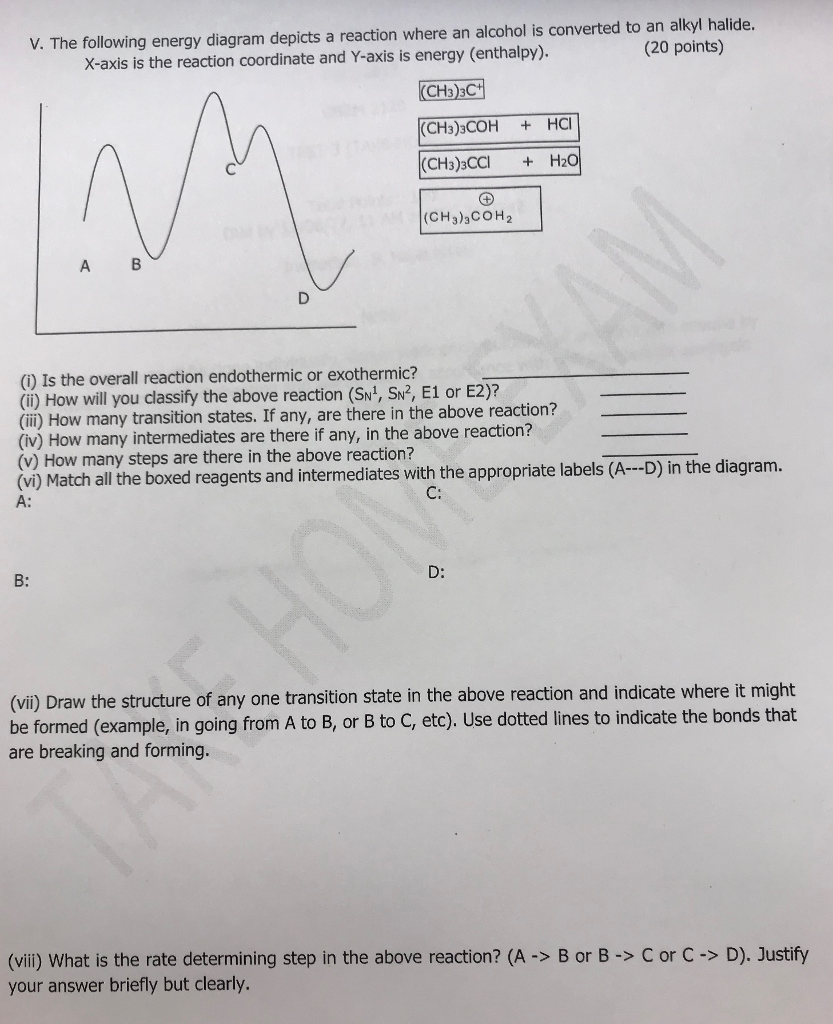
39 use the energy diagram for the reaction to answer the questions.
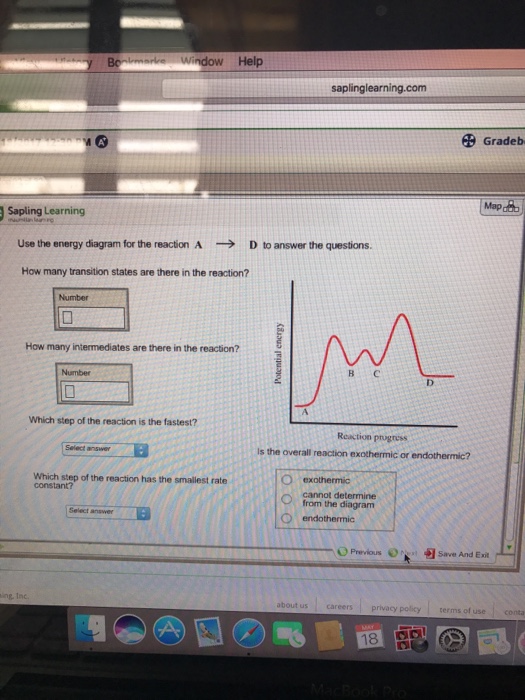
Hi Folks! With MCU’s Loki premiering this week, I thought it would be good to talk about and time travel in the MCU, and perhaps try to clear up the misconceptions around Avengers: Endgame. Loki’s head writer Michael Waldron has said that a ton of effort was put into solidifying the MCU’s rules for time travel in the series, and they expect it to stand up to scrutiny and answer any outstanding questions and controversies. I am anxious myself to see whether or not this is true, and I expect som... Transcribed image text: ted 4 out of 40 Use the energy diagram for the reaction A - D to answer the questions How many transition states are there in the ...
Alex didn't know what to feel. He had been among the other technicians for long enough to get to know a lot of them, but not to consider them genuine friends. He had only thought of them as coworkers, but he had enjoyed their company. He tried to tell himself it was just like changing jobs, or even moving. He hadn't seen them die, but that didn't change anything. With an old job you could go back and ask how things were, but there was no going back now. When Eric broke the news, he put a good d...
Use the energy diagram for the reaction to answer the questions.
######Preface Pleasantries, pleasantries. Just want to say a few things *real quick* since first post. My main motivator in life has been my unresolved anger issues. I tell you this because I want you to know if seeing blatant manipulation makes you hotter than a two-dollar pistol, even when you know you're holding blank checks to some of the biggest bank accounts in the world, I hear you — I seem to break after GME [goes down $20 in a day](https://i.imgur.com/2ublvVH.png). Also not a writer, yo... Welcome back you Sexy Space dudes, chicks, and space faring individuals that do not fit within the confines of our primitive binary system. You are readin A Tour Down Under, a story set in the Sexy Space Babes universe created by the masterful u/Bluefishcake and not myself. I'm back with part B of this chapter y'all so grab a hot or cold beverage, get a snack if your snackish, sit or lay down, relax and get ready to read. Just before we get started let me acknowledge the awesome beta readers o... I don’t have a fear of flying; I have a fear of Phil. Who’s Phil? Phil is the guy who tightens the bolts that hold the wings onto the plane, the critical ones that keep them from ripping off in mid-flight, sending it hurtling to the ground in a pirouette death spiral. Some time ago, Phil got distracted and left one of those wing bolts about a quarter-less tightened than he should. Normally Phil doesn’t do that; he’s good at his job, takes a lot of pride in it. Maybe he received an ill-timed te...
Use the energy diagram for the reaction to answer the questions.. Use the following energy profile diagram to answer Questions (a) to (c) below. (a) From the diagram, determine the value of the; (i) enthalpy of the reaction (ii) activation energy of the reaction. (b) State whether the profile diagram is for an endothermic reaction or an exothermic reaction. Minimal analysis welcome! You're very welcome to judge me with stereotypes. Have been in typology for 3+ years, and never quite related to any type's general image. • Describe your upbringing. Did it have any kind of religious or structured influence? How did you respond to it? Not too bad, I had the freedom to develop as I did. I got into decent schools though I lament not doing better. For the most part I was a loner and socially insecure, compensated by valuing independence and projecting c... ######Preface Pleasantries, pleasantries. Just want to say a few things *real quick* since first post. My main motivator in life has been my unresolved anger issues. I tell you this because I want you to know if seeing blatant manipulation makes you hotter than a two-dollar pistol, even when you know you're holding blank checks to some of the biggest bank accounts in the world, I hear you — I seem to break after GME [goes down $20 in a day](https://i.imgur.com/2ublvVH.png). Also not a writer, yo... Transcribed image text: Use the energy diagram for the reaction AD to answer the questions. How many transition states are there in the reaction?
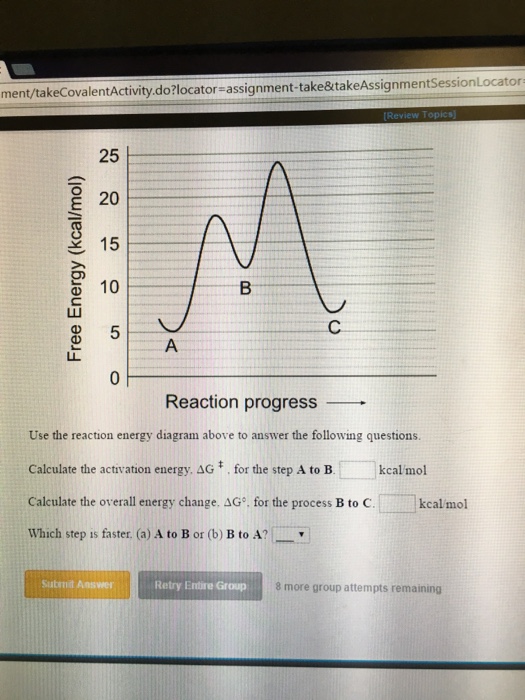
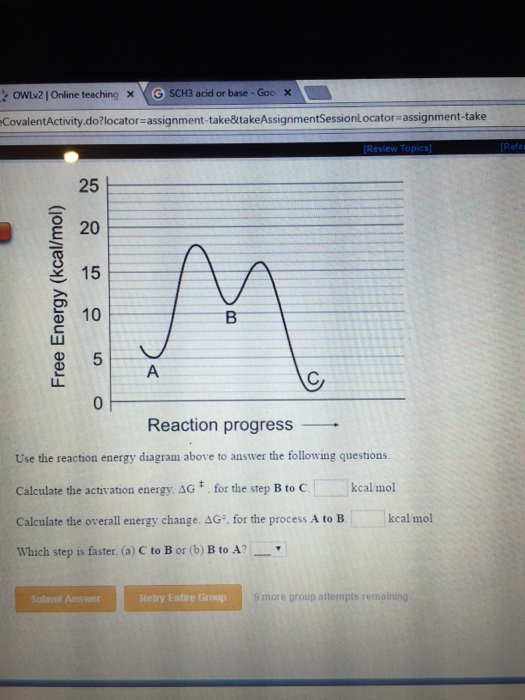
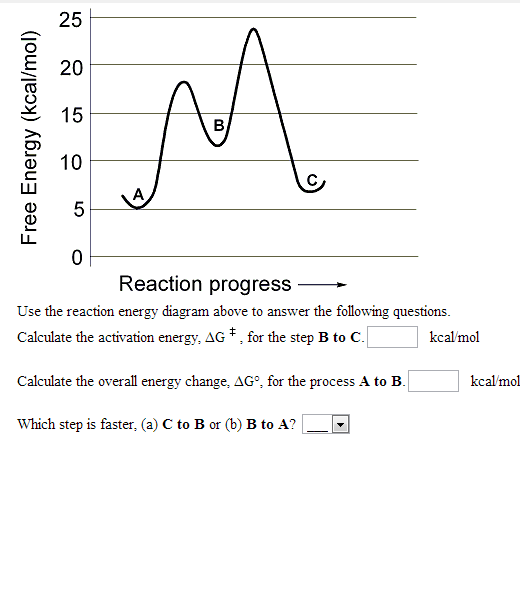
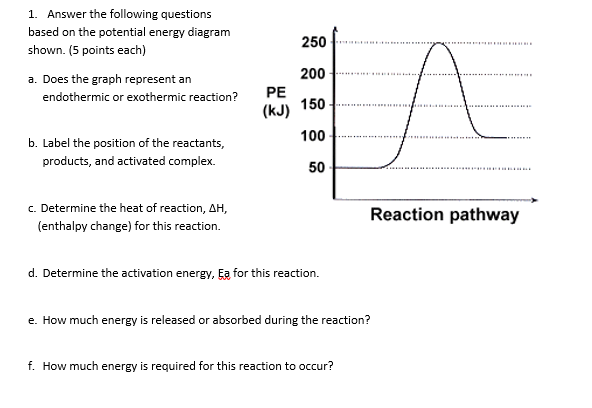
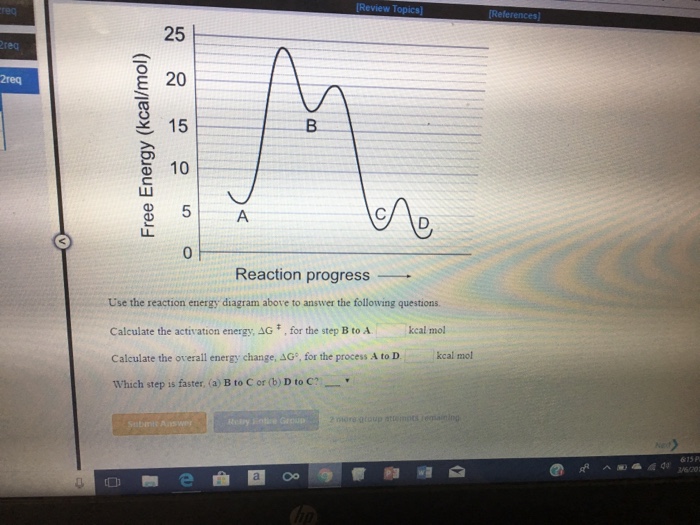
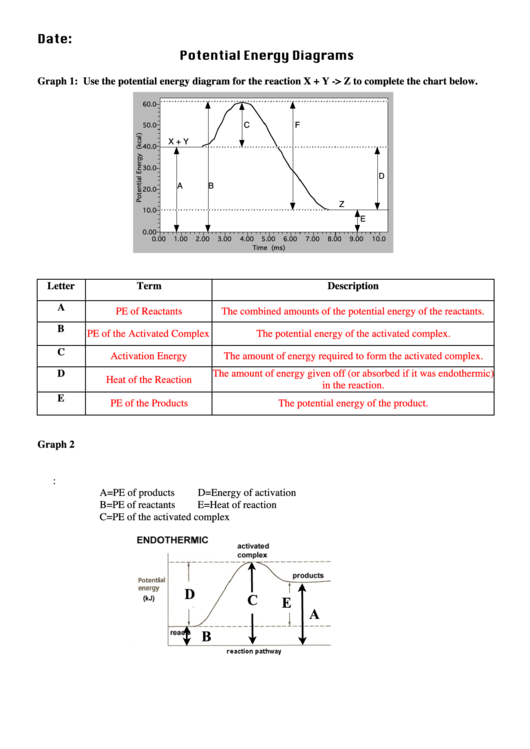
Note: I take Pure Chem + Bio. All Physics tips are courtesy of my classmates and this community ([https://www.reddit.com/r/SGExams/comments/qqr4pn/o\_levels\_pure\_physicscombined\_science\_p1\_tips/](https://www.reddit.com/r/SGExams/comments/qqr4pn/o_levels_pure_physicscombined_science_p1_tips/)) ​ 3rd last exam day till the end. Might be your last day for some. Either way, hope you had a good rest yesterday. Pure Physics starts at 8am, so make sure to head to school earlier. &a... View peds mmm.doc from AA 1Potential Energy Diagrams USE THE POTENTIAL ENERGY DIAGRAM TO ANSWER THE QUESTIONS BELOW: 1. Is the overall reaction as shown exothermic or endothermic? Endothermic 2. What Free Energy (kcal/mol) B. 25 20 15 10 Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, AG, for the step B to C. kcal/mol Calculate the overall energy change, AG°, for the process A to B. kcal/mol Which step is faster, (a) C to B or (b) B to A? Free Energy (kcal/mol) B. Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what determines the rate at which the reaction occurs?
Name _____ Potential Energy Diagrams Use the following potential energy diagram to answer the questions below. 1. Is the overall reaction as shown exothermic or endothermic?_____ 2. What is the activation energyfor the forward reaction?_____ 3. # Why is A Level Chemistry So Hard? If you are taking A Level Chemistry, you will probably agree, like most students, that A Level H2 Chemistry is difficult and you have good reasons to do so. The concepts are complex and involve much memory work. There is a steep increase in the learning curve. This is only natural. Having now graduated from the top 20% of the O Level cohort, the syllabus is now made much tougher to further differentiate among all of you. # Much Memory Work is Required C... Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ΔG, for the step A to B. kcal/mol. Calculate the overall energy change, ΔG°, for the process B to C. kcal/mol. Which step is faster, (a) A to B or (b) C to B. fullscreen Expand. Question: D to answer Use the energy diagram for the reaction A the questions. How many transition states are there in the reaction? transition states: ...
Chemistry 12 Unit 1-Reaction Kinetics Worksheet 1-2 Potential Energy Diagrams Page 1 Chemistry 12 Worksheet 1-2 - Potential Energy Diagrams USE THE POTENTIAL ENERGY DIAGRAM TO ANSWER THE QUESTIONS BELOW: 1. Is the overall reaction as shown exothermic or endothermic? _____ 2. What is the activation energy for the forward reaction?
[**Beginning**](https://www.reddit.com/r/PerilousPlatypus/comments/9wm9ha/wp_the_sol_system_was_an_experiment_by_aliens_to/) **|** [**Previous**](https://www.reddit.com/r/PerilousPlatypus/comments/kmbz84/serialuwdff_alcubierre_part_74/) An overwhelming sense of revulsion welled up within Kai as the story of the Amalgans unfurled in his consciousness. Neeria had not kept the information from him before, she had simply not seen any purpose to disclosing it. Until the moment the interlopers had ap...
Rates, Temperature and Potential Energy Diagrams Worksheet Part 1: 1. Use the potential energy diagram shown to the right to answer the following: a. Label the axis. yaxis is potential energy (kJ or kJ/mol) xaxis is reaction progress b. What does each curve represent? Each curve represents a
Potential energy. Use the energy diagram for the reaction A → D to answer the questions. How many transition states are there in the reaction? transition states: B How many intermediates are there in the reaction? A D Reaction progress intermediates: Which step of the reaction is the fastest? Potential energy.
Use this energy diagram to answer these questions. 1. The enthalpy of the reactants of the reaction is about kilojoules. 2. The enthalpy of the products of the reaction is about kilojoules. 3. The activation energy of the reaction is about kilojoules. 4. The heat of reaction (ΔH) of the reaction is about kilojoules.
Just learned this morning that I passed PE Chemical! I'll share my experience here for others in case it's helpful. Some brief background on me: I got my B.S. in chemical engineering in 2016, so I've been out of undergrad for 5 years. I took and passed the FE exam in 2016, around graduation time. My current employment is in energy/power regulation for the state, and I've been in this position for the past few years. I chose to take the PE Chemical exam since I was most familiar with it. I hit ...
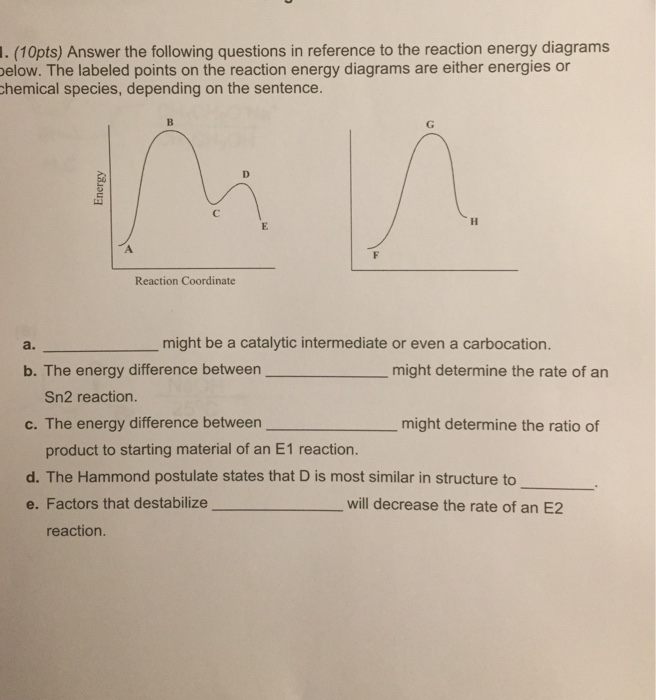
Determine which of the two diagrams here (both for the same reaction) involves a catalyst, and identify the activation energy for the catalyzed reaction: Answer: Diagram (b) is a catalyzed reaction with an activation energy of about 70 kJ.
D to answer Use the energy diagram for the reaction A the questions. How many transition states are there in the reaction? transition states: Potential energy How many intermediates are there in the reaction? intermediates: Reaction progress Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant?
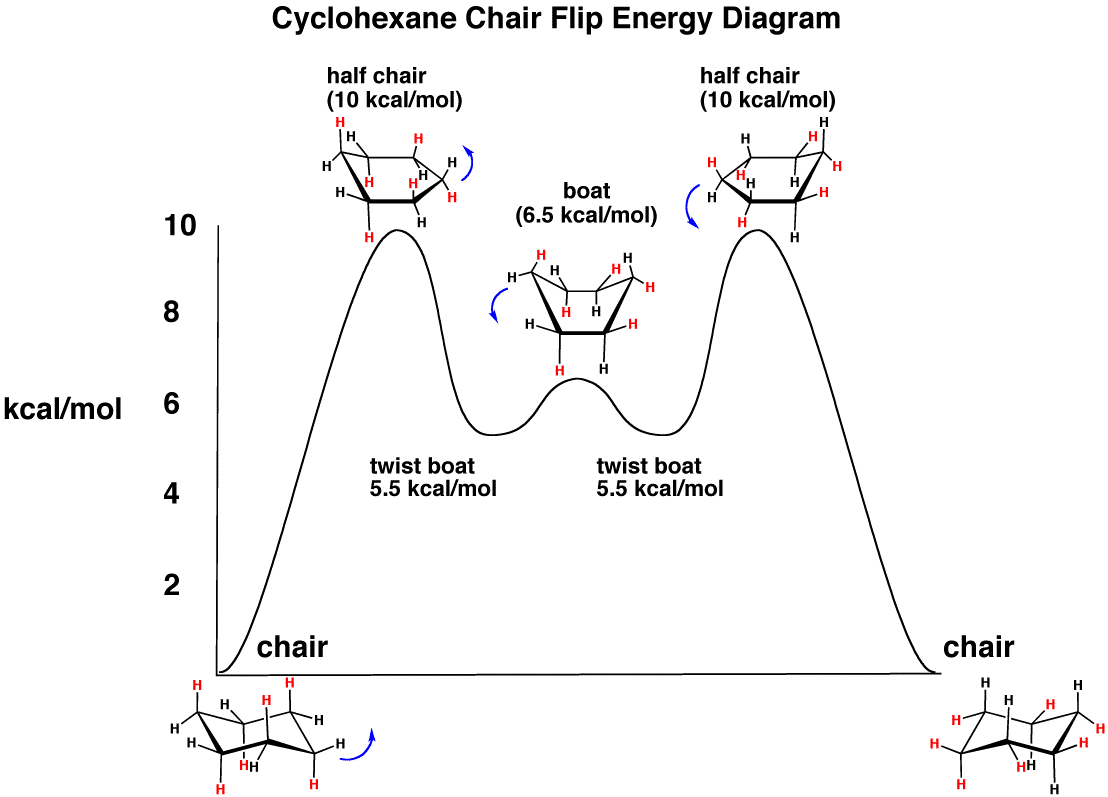
Use the energy diagram for the rearrangement reaction of methyl isonitrile to acetoni- trile to answer the following questions. 7. What kind of reaction is represented by this diagram, endothermic or exothermic? 8. What is the chemical structure identified at the top of the curve on the diagram? Cor«plc¥ 9. What does the symbol E represent? 10.
Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? What is the activation energy of the reaction? FREE Expert Solution. Recall that an energy diagram is usually read from left to right. The components of a one-step energy diagram are:
15. Show the AH, the Activation Energy for theforward reaction and the Activation Energy for the reverse reaction on the graph above. 16. As reactant particles approach each other before a collision, the Potential Energy goes ... Use the following Potential Energy Diagram to answer the questions below: Progress of Reaction $0 - 12 a) Determine ...
The figure above represents the reaction profile of a two step, exothermic reaction. The y-axis represents the potential energy of the reaction species, and the x-axis represents the progress of the reaction. The reaction is exothermic because the energies of the products are lower than those of the reactants. The reactants are represented by the horizontal line at the far left of the graph ...
This is moving on a glacial pace, I´m really sorry for this and I hope you are still interested on the project. This things was supposed to go out last week but life happened again. ​ I tried to shorten up a bit the descriptions this time. I think I failed miserably at it, but if you could see the original lenght of the post it would be very clear how much this is leaner... It was a real mess before the cutting floor. ​ So, what do you think? ​ \---- Pas...
Alright y'all, ​ I've had a few Sailor Jerry's and am bored so I figured I'd do a write-up and share some DD on a play that I'm big on. ACU - Aurora Solar Technologies. ​ Here's a great play in the solar energy renewables space that in my opinion is still very undervalued. I believe there'll be a lot of tailwinds coming up for green energy in general and the solar industry has been growing at 25% YoY for the last 2 decades with no signs of stopping. ​ We'...
In this reaction, the total energy of the reactants is 80 kJ mol-1, the total energy of the products is -90 kJmol-1 and the activation energy for the forward reaction is 120 kJ mol-1. a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic.
Neon Genesis Evangalion Reaction Log Bath Scenes: III S1 E16 /21/21 intro looks neat. why do they have the alchemy tree like in FMAB? and what's with all these silhouettes of nude ladies? guess japan just be like that. wow all this ordinace going into god is cool. shot of the long blue-haired ladies' ass. short-haired blond lady in sexy swimsuit. wait, he can j...
Transcribed image text: Use the energy diagram for the reaction AD to answer the questions. How many transition states are there in the reaction?
Use this energy diagram to answer these questions. 1. The heat content of the reactants of the forward reaction is about kilojoules. 2. The heat content of the products of the forward reaction is about kilojoules. 3. The heat content of the activated complex of the forward reaction is about kilojoules. 4.
Consider the potential energy diagram shown below. This graph shows the chemical potential energy in a reaction system over time. The y-axis is potential energy in kilojoules. The x-axis is the reaction progress, or time. Does this graph represent an endothermic or an exothermic reaction? Explain your answer.
Question 15. SURVEY. 30 seconds. Q. How much energy is released when two moles of methane (CH 4) reacts at 298 K and 101.3 kPa according to the following equation: CH 4 (g) + 2O 2 (g) → CO2(g) + 2H2O (l) answer choices. 890.4 J. 890.4 kJ.
25 E 20 é 15 C D 0 Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, Î G , for the step A to B. Calculate the overall energy change, AGo, for the process B to C.
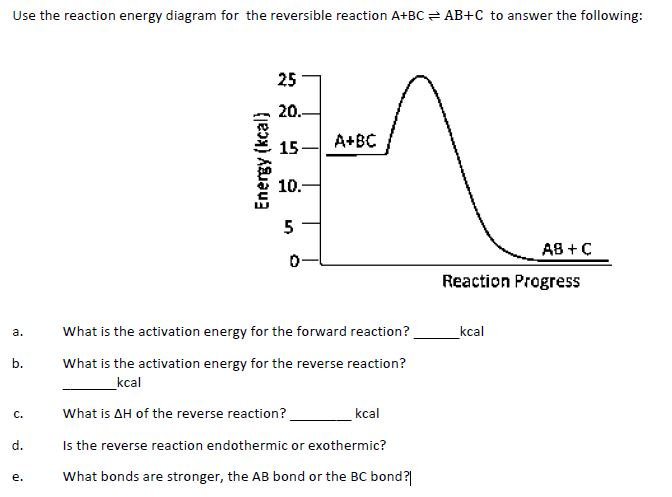
Here is an energy diagram for the reaction: Use the energy diagram to answer these questions. Can you determine the activation energy of the reverse reaction? C + D → A + B. Learn this topic by watching Energy Diagram Concept Videos ... Frequently Asked Questions
Use the energy diagram for the reaction A D to answer the questions. How many transition states are there in the reaction? transition states: Potential energy How many intermediates are there in the reaction? intermediates: Reaction progress Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant?
Hey everyone, I’m Bill and I’m currently in my 2nd year of a BBiomed single degree here at Monash. A few months ago, I’ve put up an expression of interest for a biomed + chem study skills post à la allevana and luneax, but man, I’ve been swamped with uni work for the past 9 weeks (thank you very much Monash for that 3/4 sem break /s). I’ll write a quick paragraph on every single subject I did, but mainly focusing on pitfalls to beware of because these are probably the most valuable for future st...
Transcribed image text: macmilan learning Use the energy diagram for the reaction A → D to answer the questions. How many transition states are there in ...
***\*Reposting here for anyone interested.\**** Just learned this morning that I passed PE Chemical! I'll share my experience here for others in case it's helpful. Some brief background on me: I got my B.S. in chemical engineering in 2016, so I've been out of undergrad for 5 years. I took and passed the FE exam in 2016, around graduation time. My current employment is in energy/power regulation for the state, and I've been in this position for the past few years. I chose to take the PE Chemica...
[Cover](https://ibb.co/JKF4vwK) || [First](https://www.reddit.com/r/HFY/comments/e30q57/the_heartless_ranger_chapter_1/) [Previously](https://www.reddit.com/r/HFY/comments/r5udd9/the_heartless_ranger_chapter_29/) [Story Discord](https://discord.gg/X6FT4ahk) (expires in 7 days) O-O-O AN: Merry Christmas (if you celebrate it)! This ain't particularly Christmassy, but I hope you guys will enjoy this gift from me to you all. Have a great day today, and take care of yourselves. O-O-O *1550, 29 ...
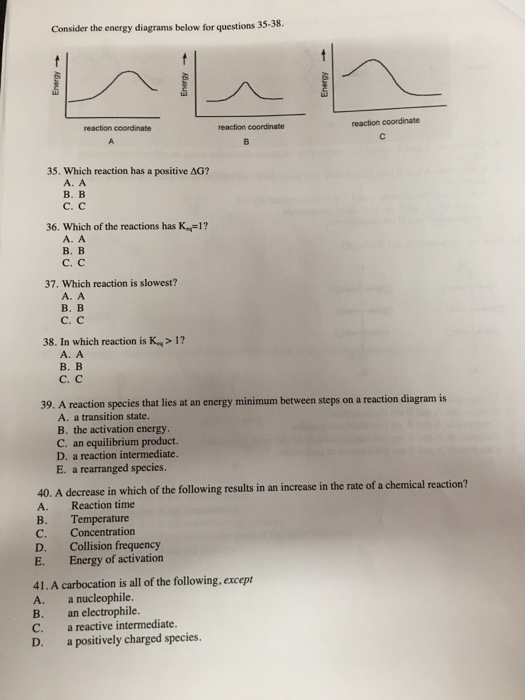
When you [last heard from me]( https://www.reddit.com/r/Parahumans/comments/l1h96y/practitioner_parenting/), I talked about Mrs. Ferguson, and her treatment of her son. In that post, I mentioned that there is a difference between being kind of a shitty parent, and being outright abusive, and that Mrs. Ferguson often straddles that line. Brett Hayward does not straddle that line. Brett Hayward is an abuser. This is not, I hope, something you need me to tell you, at this point. So, if you all kn...
Use the energy diagram for the reaction A D to answer the questions. How many transition states are there in the reaction? How many intermediates are there in the reaction? Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant? Assume the frequency factor (?) is the same for each elementary reaction.
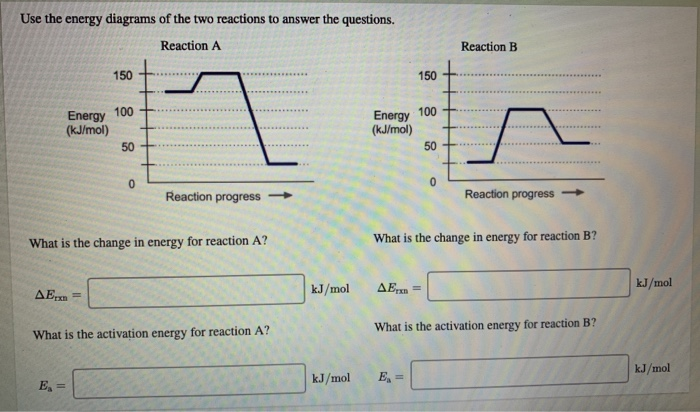
Question: Use the energy diagrams of the two reactions to answer the questions. Reaction A Reaction B 150+ Energy 100 (kJ/mol) HH Energy (kJ/mol) 50 TH +..... Reaction progress Reaction progress- What is the change in energy for reaction A? What is the change in energy for reaction B? kJ/mol AE,an = kJ/mol AE What is the activation energy for reaction B? What is
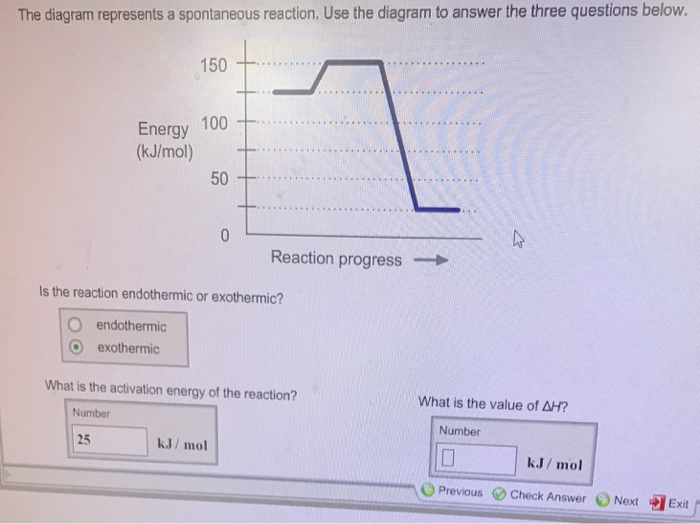
The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. a. Is the reaction endothermic or exothermic? b. What is the activation energy of the reaction?
Problem Details. Use the energy diagram for the reaction A → D to answer the questions. Q. Which of the following has ΔG°f = 0 at 25°C?a. O3 (g)b. O (g)c. H2O (g)d. H2O (l)e. Na (s) Q. Predict the sign of ΔG for an endothermic reaction with adecrease in entropy. a)cannot predict b)positive c)negative d)no change e)Aquarius.
Use the enthalpy diagram on the right to answer the following questions. Which arrow(s) represent endothermic reactions? Which arrow represents the overall reaction? Is the overall reaction endothermic or exothermic?
Transcribed image text: Use the energy diagram to answer questions about the reaction and mechanism. + Step 1: NO2(g) + F2 (g) NO2F (g) +F () Step 2: NO2 ...
Transcribed image text: Use the energy diagram for the reaction A D to answer the questions How many transition states are there in the reaction?
# This is Part 2, if you have not read [Part 1](https://www.reddit.com/r/UFOs/comments/qalb6w/part_1a_discussion_why_tom_delonge_lue_elizondo/?utm_source=share&utm_medium=web2x&context=3), I recommend doing so first. https://preview.redd.it/eljc90y727u71.jpg?width=1596&format=pjpg&auto=webp&s=e6db08984d22041f7b896c05b9f1dea06803335f # 8. The idea that consciousness is somehow related to this phenomena was my presumption, as I’m sure it has been for a lot of you. However, m...

Nuclear powerplant in Belgium Please mention me on Instagram: @Fredpaulussen or link to my website fredography.be Thank you!
While there may only exist 9 Enneagram types, all Enneagram teachers have sought to give nuance and variation to the 9 types through the expansion of the types themselves, in order to account for differences of individuals in the same types. Some ideas include: * [Claudio Naranjo's 27 Instinctual Subtypes](https://www.integrative9.com/enneagram/27-subtypes/) * [Katherine Fauvre's Tritypes](https://www.katherinefauvre.com/tritype) * [The Enneagram Institute's 18 Riso-Hudson Subtypes](https://www...
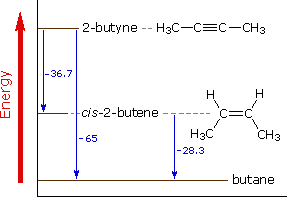
O were to decompose via reaction 4, approximately how much energy would be released or absorbed? (A) 65 kJ of energy will be absorbed. (B) 65 kJ of energy will be released. ... Use the following diagram to answer questions 13-15. Reaction Coordinate Potential Energy 1 2 3 4
​ | \---------------------------------Table of Contents-------------------------------------| |:-| |[Chapter 24](https://www.reddit.com/r/The_Guardian_Temple/comments/r59mqv/of_nite_and_dei_book_2_chapter_24/) l [Chapter 25](https://www.reddit.com/r/libraryofshadows/comments/rahlnv/of_nite_and_dei_book_2_chapter_25/)| ​ Sellenia froze, looking around the dimly lit corridors painted red by the emergency lights. “*Soardoria?! What’s wrong?*” Sellenia called out in her min...














0 Response to "39 use the energy diagram for the reaction to answer the questions."
Post a Comment