40 rubidium electron dot diagram
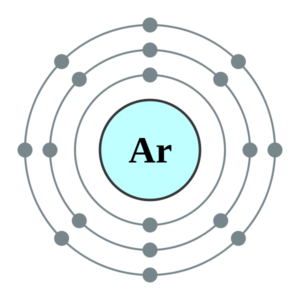
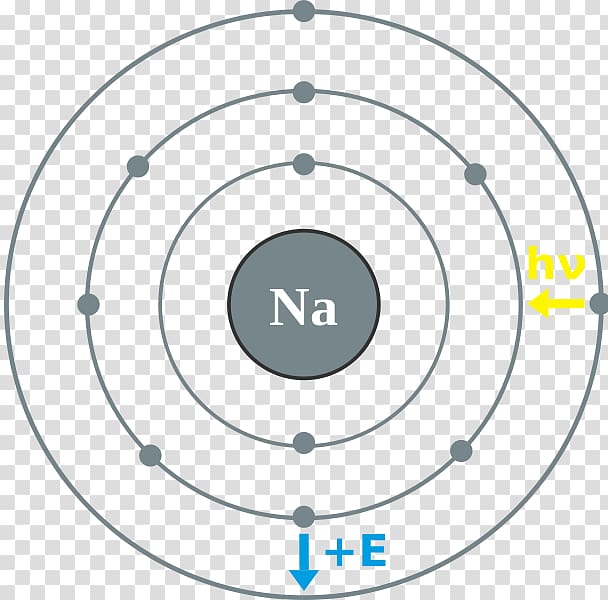
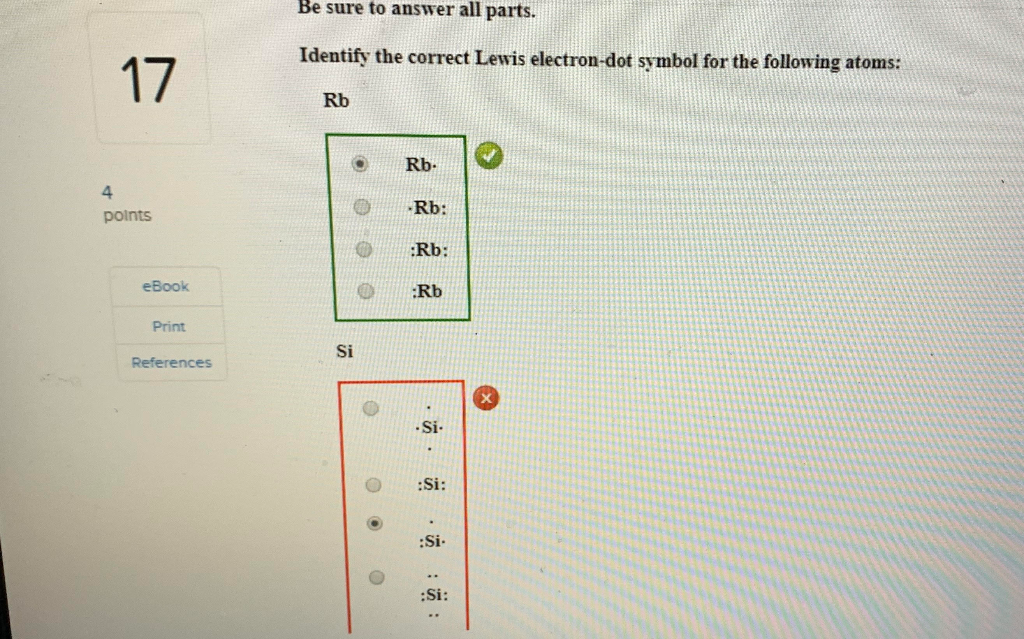
1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of .. Rb+, Se2−. The first orbital (an s orbital) can contain only two electrons.. Rubidium. This diagram of a rubidium atom shows the electron shell.
English: Lewis dot diagram for rubidium. Date: 30 September 2011: Source: Own work: Author: Adrignola: Licensing . I, the copyright holder of this work, hereby publish it under the following license: ...
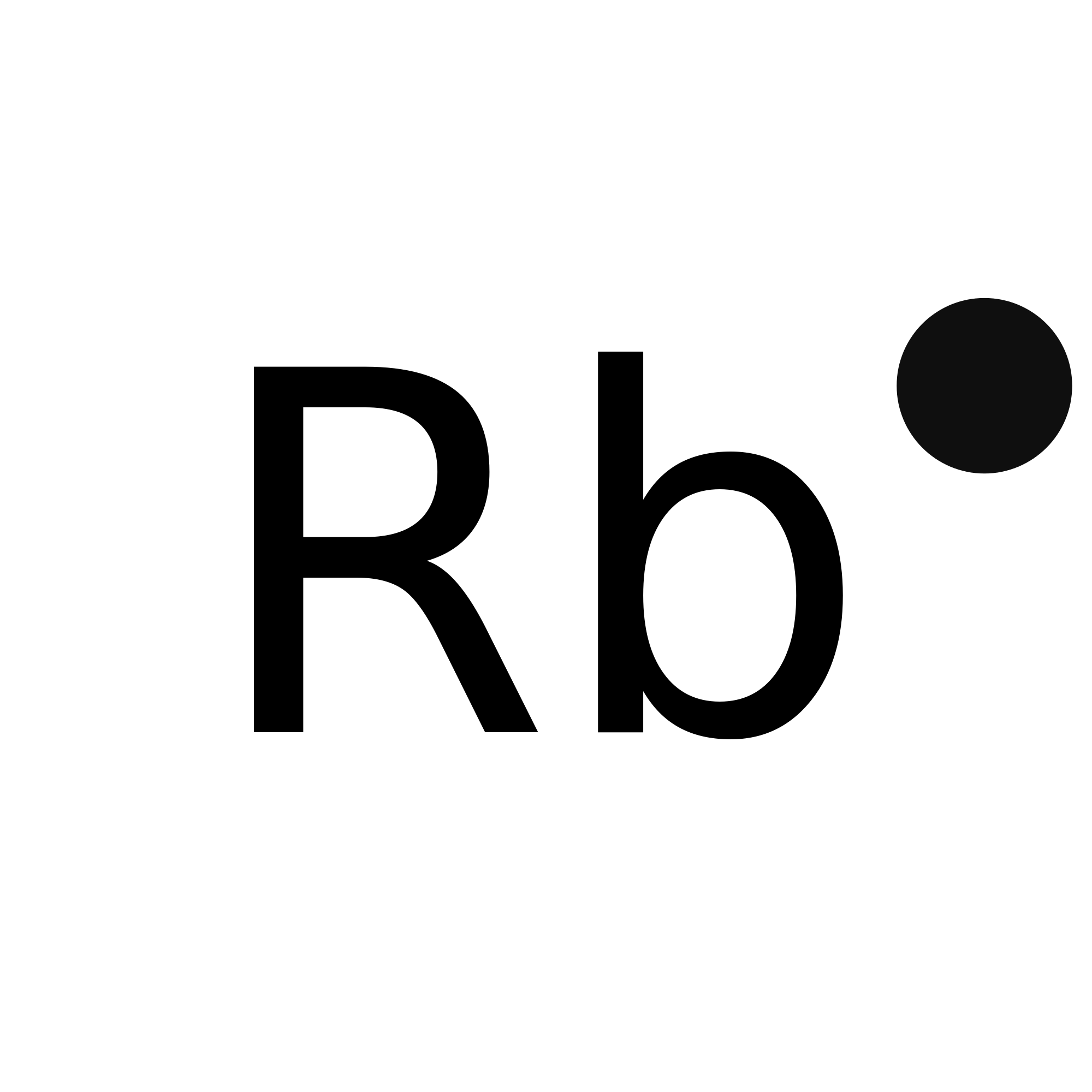
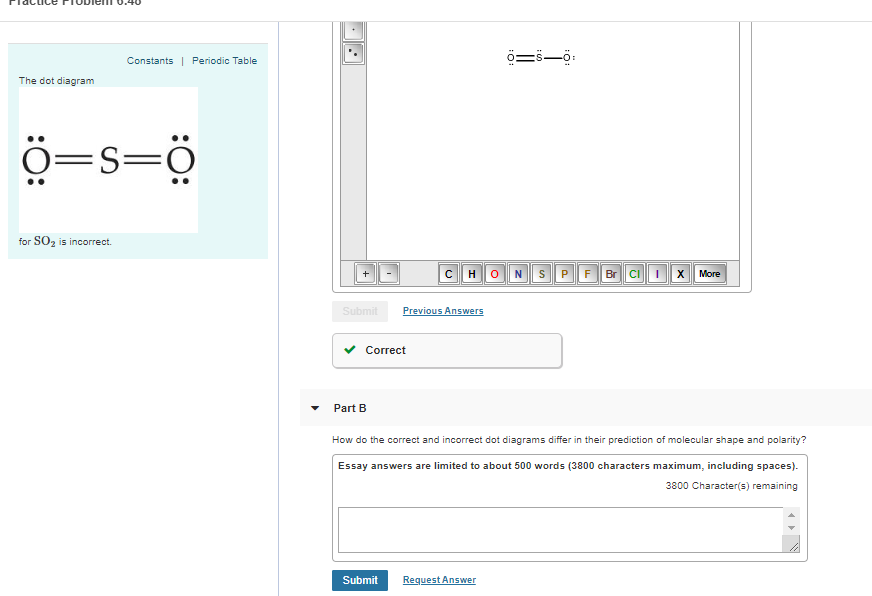
Rubidium electron dot diagram
in an electron dot diagram or rubidium, there is one dot. in an electron dot diagram of silicon, there are four dots. which element would you expect to be more reactive? group 7A. in a periodic table hay included electron dot diagrams, in which column would the diagrams contain more dots-- GROUP 2A or Group 7A. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Lewis Dot Diagrams of Atoms Select the correct electron dot diagram for an atom of each of the elements: rubidium, selenium and astatine. At Rb: Se: At: Rb Se Se At Rb Se: Rb: :At: Se: At Rb: At Se: Rb: At: Se Rb Se Rb At: Tries 0/5 Submit Answer Send Feedback Post Discussion NOV OOO
Rubidium electron dot diagram. Rubidium atoms have 37 electrons and the electronic shell structure is [2, 8, 18, 8, 1] with Atomic Term Symbol (Quantum Numbers) 2S1/2. Atomic Number, 37.Electronic Shell Structure: 2, 8, 18, 8, 1Atomic Number: 37Electronic Configuration: 5s1CAS Number: CAS7440-17-7 In Section 9.1 "Lewis Electron Dot Diagrams", we saw how ions are formed by losing electrons to make cations or by gaining electrons to form anions.The astute reader may have noticed something: Many of the ions that form have eight electrons in their valence shell. Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or ... For the Rb+ structure use the periodic table to find the total number of valence electrons for Rb. Once we know how many valence electrons there are in Rubid... Rubidium is a chemical element with atomic number 37 which means there are 37 protons and 37 electrons in the atomic structure.
Draw the electron dot structure for each element in Question 7. How many electrons will each element gain or lose in forming an ion? Calcium. Fluorine. Aluminum. Oxygen. Write the name and symbol of the ion formed when: Potassium loses one electron. Zinc loses two electrons. Fluorine gains one electron 9.Rank the atoms from lowest to highest ionization energy a) Cl, Br, Ar b) K, Rb, Na c) C, N, O d) Rb, Ba, Sr . 10. Write the short hand electron configuration for element 19, element 53, element 88. Which families do they belong to and give electron dot structure. 11. Specify properties of m, nm, sm. On a test we were supposed to draw a valid Lewis dot structure for rubidium iodide, not necessarily the correct Lewis dot structure. I drew Rb::::I. Since there is one valence electron from Rb and seven from I, I figured this makes sense and satisfies the octet rule. Structure, properties, spectra, suppliers and links for: Rubidium, Rubidium hydride, 13446-75-8.
lewis dot structure for rb. A. Lewis dot structure for an atom of chlorine is The number of valence electrons for an atom. Rb Ar C-4 Ca+2 S Cl-1 Fr+1 O-2 ©POGIL - 2005 4/6 Authored by: Lizabeth M. Lewis Dot Structures of Atoms and Ions. Learning activities help people succeed - find your success here. In an electron dot diagram of rubidium, there is one dot. In an electron dot diagram of silicon, there are four dots. Which element would you expect to be more reactive? 3. Potassium, an alkali metal, and bromine, a halogen, are both in Period 4 of the periodic table. Which element has a higher Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. The dot structure for Rubidium is Rb with a dot on the top right of b. Rb is the short form of rubidium. Draw electron dot diagrams for elements. An electron dot diagram is like a football diagram. How do we show electrons in atoms? Diagrams contain a lot of ...Nitrogen: 1 s 2 2 s 2 2 p 3Neon: 1 s 2 2 s 2 2 p 6Lithium: 1 s 2 2 s 1Beryllium: 1 s 2 2 s 2Missing: rubidium | Must include: rubidium
Sources of Rubidium: Occurs abundantly, but so widespread that production is limited. Usually obtained from lithium production. Uses of Rubidium: Has limited commercial uses and is primarily used for research purposes. Additional Notes: Rubidium Menu. Rubidium Page One. Overview of Rubidium; Rubidium's Name in Other Languages; Atomic Structure ...
The dot structure for Rubidium is Rb with a dot on the top right of b. Rb is the short form of rubidium. The dot structure as you know can only be a max of 8 and dots are added counterclockwise.
The dot structure for Rubidium is Rb with a dot on the top right Rb is the short form of rubidium. know can only be a max of 8 and dots are added counterclockwise. Anyway, a good counting place on...
Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
lithium, sodium, potassium, rubidium, cesium. Place the elements above in order from smallest energy needed to remove the outermost electron to the largest energy needed to remove the outermost electron: ... Sulfur Electron Dot Diagram. 6. Which elements have 5 valence electrons.
Learn the basics about Drawing electron configuration diagrams. Richard Louie Chemistry. Contact. Search. Rubidium. Electronic configuration. Electronic configuration. 1s2 2s22p63s23p63ds24p65s1. >> Back to key information about the element.Sep 02, · A step-by-step explanation of how to draw the Lewis dot structure for Rb (Rubidium).
Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is ...
Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is ...
Choose the correct Lewis Dot diagram for MgO. answer choices . Tags: Question 23 . SURVEY . 30 seconds . Q. Rubidium has a lower ionization energy than sodium. One reason for this is that the - answer choices . number of protons is increasing. number of energy levels is decreasing. valence electrons are farther from the nucleus ...
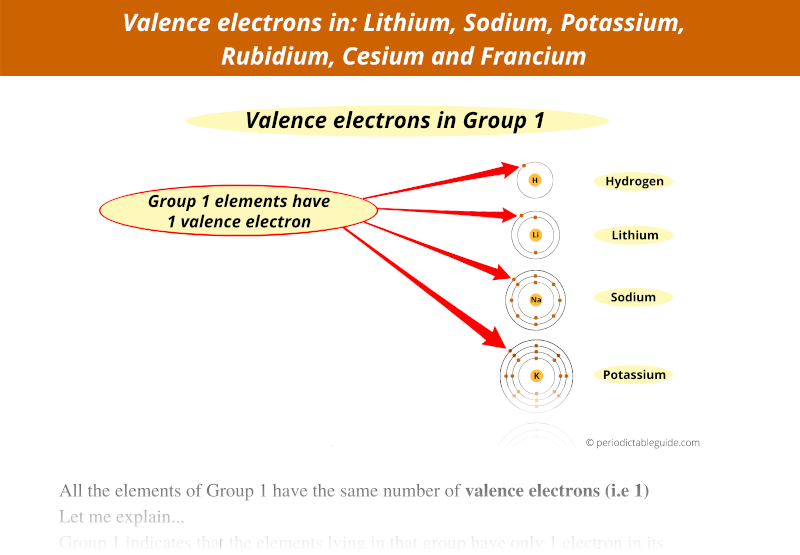
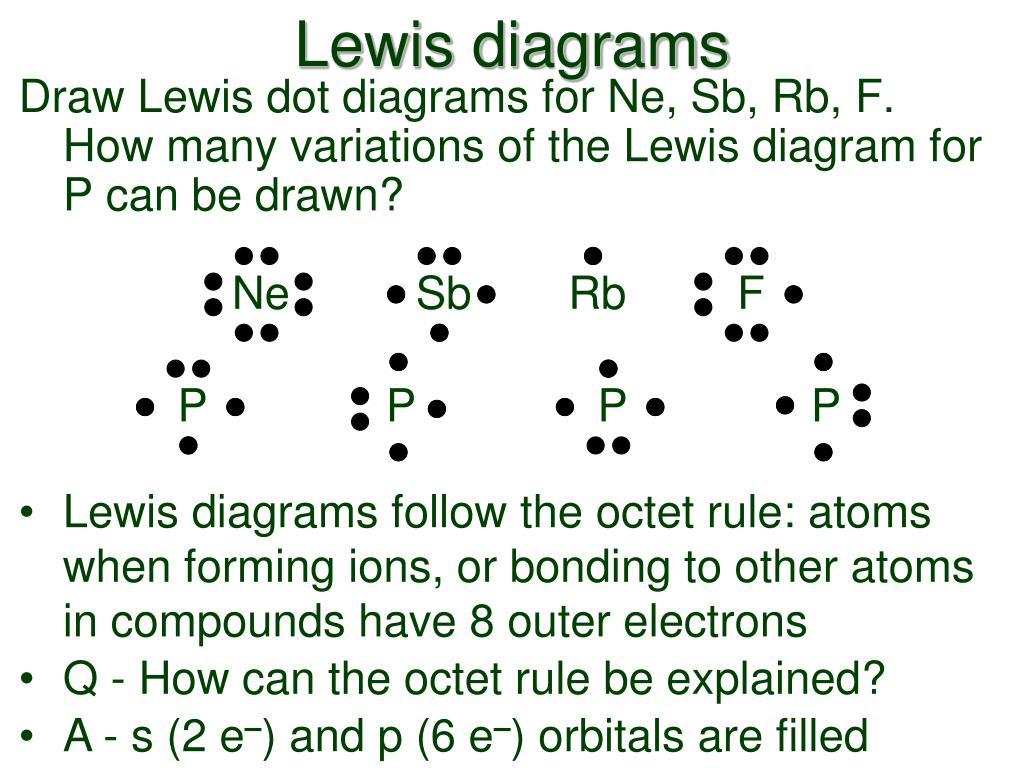
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
rubidium
oxygen
answer explanation . Tags: Topics: Question 2 . SURVEY . Ungraded . 30 seconds . Report an issue . Q. An unidentified element has many of the same physical and chemical properties as magnesium and strontium but has a lower atomic mass than either of these elements. ... A student created the Lewis dot diagram of ...With the periodic table symbol Rb, atomic number 37, atomic mass of 85.4678 g.mol-1, and electron configuration [Kr] 5s1, rubidium is a soft, silvery-white metal composed of two isotopes. It reaches its boiling point at 688°C, 1270°F, 961 K, while the melting point is achieved at 39.30°C, 102.74°F, 312.45 K.
Rubidium Overview Rubidium Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 4 s2 3 d10 4 p6 5 s1 Abbreviated Electron Configuration [Kr] 5s1 Sources Occurs abundantly, but so widespread that production is limited. Usually obtained from lithium production. Atomic Symbol Rb Uses Used as a catalyst, photocells, and vacuum and cathode-ray tubes ...
Lewis Dot Diagrams of Atoms Select the correct electron dot diagram for an atom of each of the elements: rubidium, selenium and astatine. At Rb: Se: At: Rb Se Se At Rb Se: Rb: :At: Se: At Rb: At Se: Rb: At: Se Rb Se Rb At: Tries 0/5 Submit Answer Send Feedback Post Discussion NOV OOO
1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium
in an electron dot diagram or rubidium, there is one dot. in an electron dot diagram of silicon, there are four dots. which element would you expect to be more reactive? group 7A. in a periodic table hay included electron dot diagrams, in which column would the diagrams contain more dots-- GROUP 2A or Group 7A.


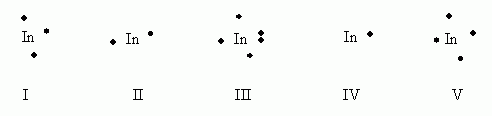







:max_bytes(150000):strip_icc()/Rubidium-58b601ee3df78cdcd83d20d6.jpg)






0 Response to "40 rubidium electron dot diagram"
Post a Comment