41 orbital diagram for potassium
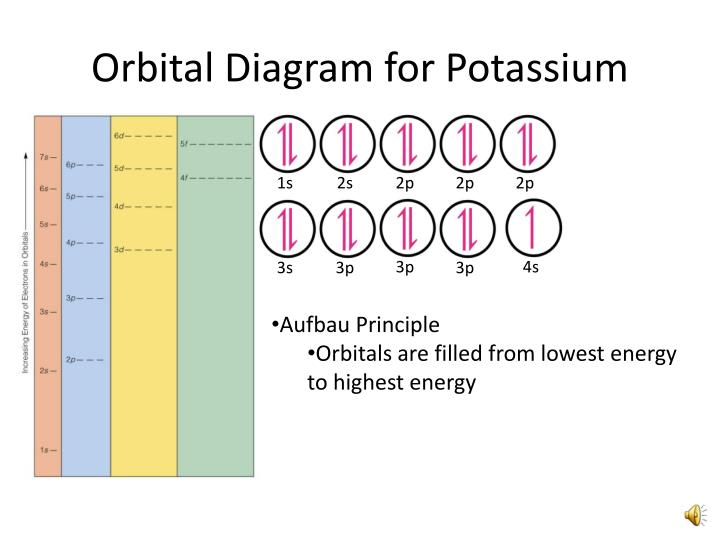
English: Orbital representation diagram depicting each orbital of an atom having its own circle within each sublevel. This diagram is for potassium. Date. 22 February 2010. Source. File:High School Chemistry.pdf, page 355. Author. CK-12 Foundation (raster), Adrignola (vector) SVG development. A Venn Diagram is a graphic organizer it can organize your data and/or information in a way that is easy... What Is The Block Diagram Of Computer And Explain It? Computer Science. Block diagram with the help of different blocks tell about the different components of the computer and... What Is The Orbital Notation Of Phosphorus? Chemistry
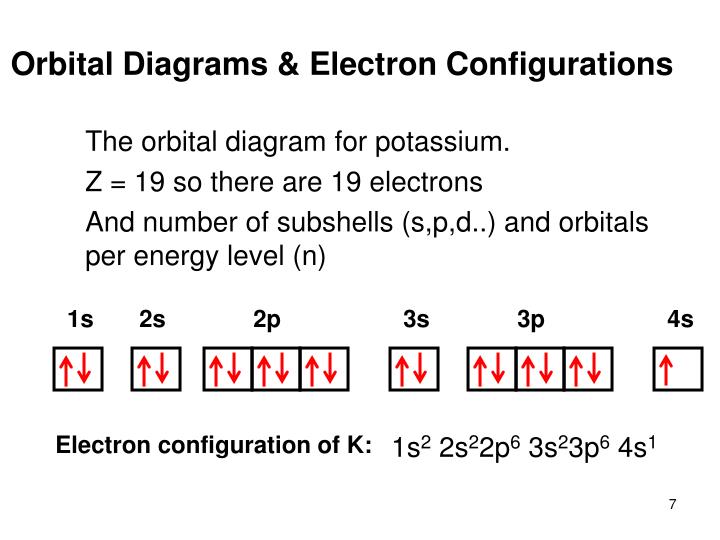
Potassium (K) ; Thermal Conductivity · 102.5 W·m·K ; Oxidation States, 1 ; Electrons Per Shell, 2 8 8 1 ; Electron Configuration, [Ar] 4s ; Orbital Diagram. 1s. ↿⇂.

Orbital diagram for potassium
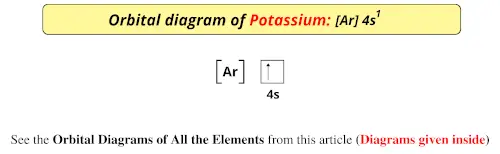
Hint :We know that the atomic number of potassium is 19. The electronic configuration of an element describes how electrons are distributed in its atomic ... Draw the orbital energy diagram for K (potassium). Represent electrons as arrows (with up or down spin). What is the core] valence electron configuration? (type in the box below) -45 Energy 2p 2s is *** -T-5 ; Question: Draw the orbital energy diagram for K (potassium). Represent electrons as arrows (with up or down spin). Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28:
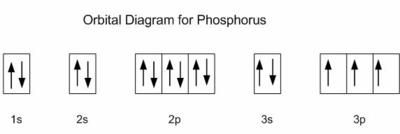
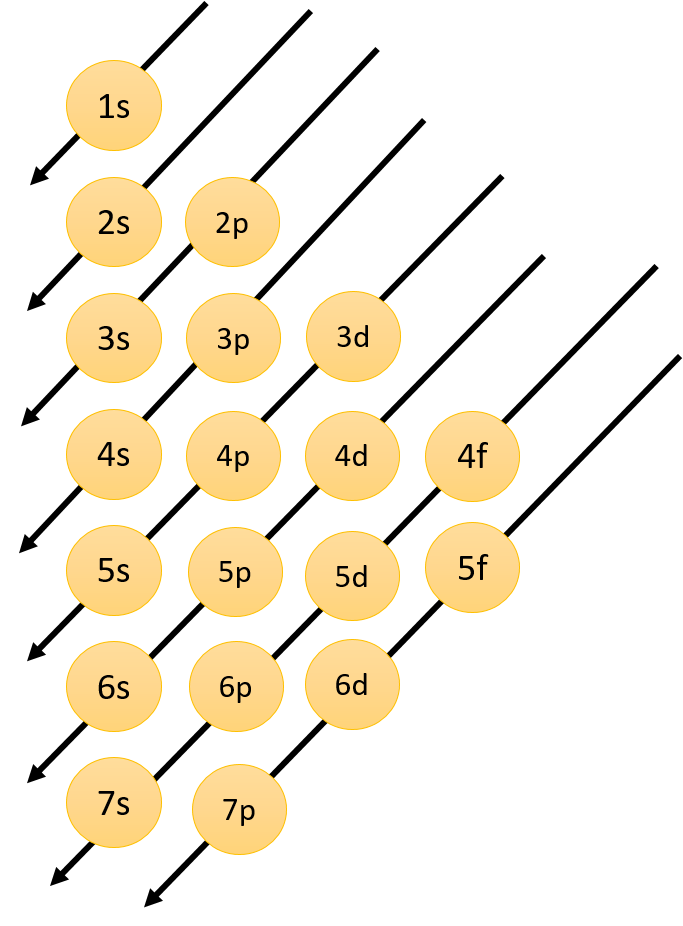
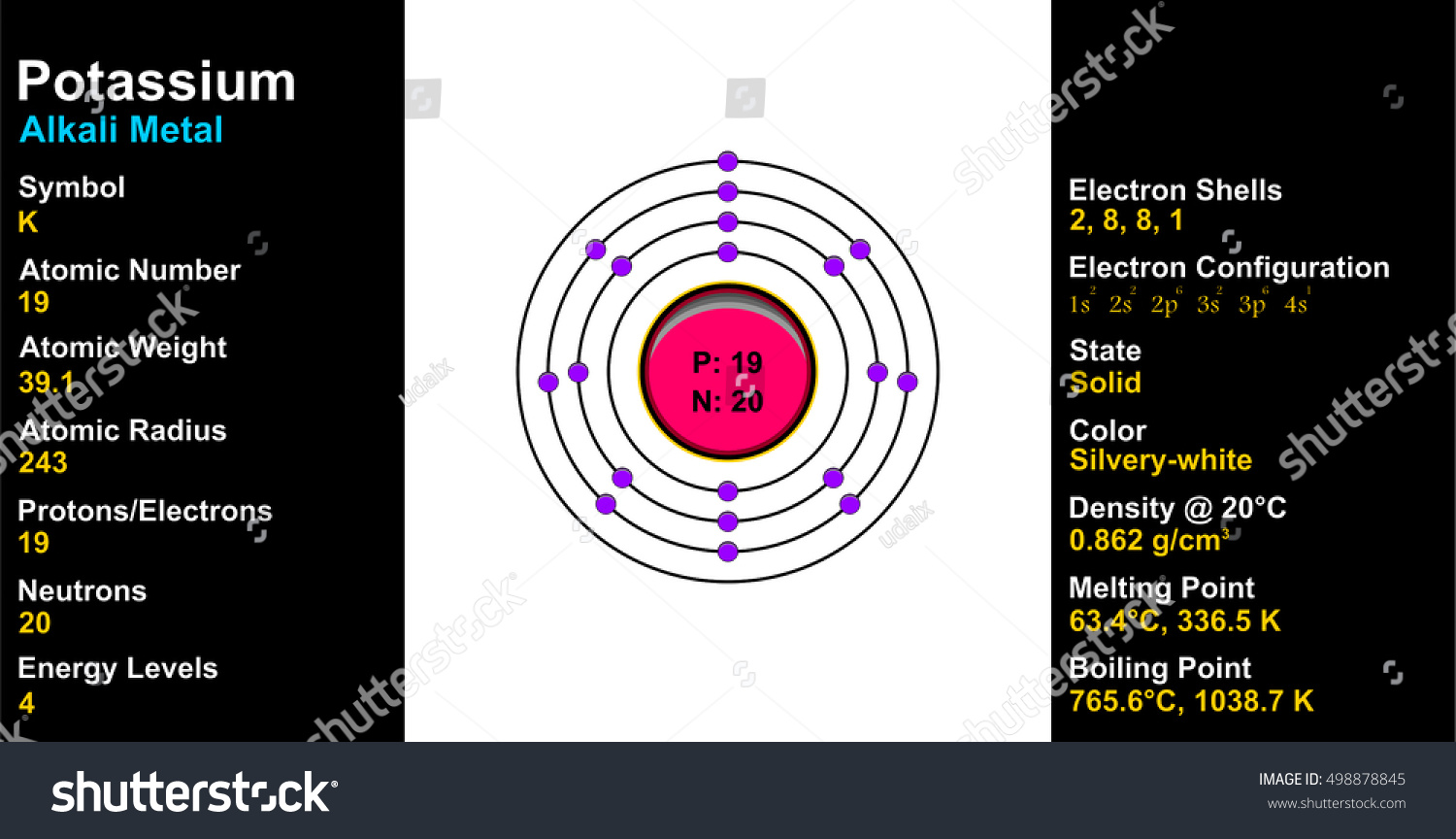
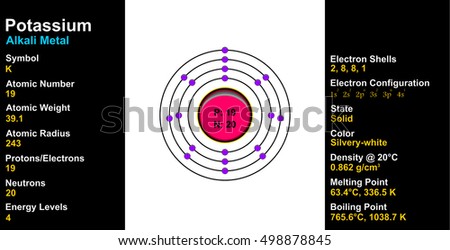
Orbital diagram for potassium. 21 Jan 2021 — As an ion potassium exists as K+, which means it loses an electron because it needs to attain stable electronic configuration and also the octet ... In writing the electron configuration for Potassium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Potassium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next ... Solution · Potassium's atomic number is 19. This means that every atom of potassium has 19 protons in its nucleus. In a neutral atom, the number of protons is ... What is orbital diagram method? An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.
So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"4s"^1". The noble gas notation is "[Ar]4s"^1". The following orbital diagram shows the increase in energy from one energy sublevel to the next, but you can write them on the same level horizontally, Electronic configuration of the Potassium atom. Valence electrons. Orbital diagram. The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second layer, and 9 on the ... Potassium(K) is the 19th element in the periodic table and its symbol is ‘K’. The electron configuration of potassium and the orbital diagram is the main topic in this article. Also, valency and valence electrons of potassium, and compound formation, bond formation have been discussed.
Orbital representation diagram potassium.svg. English: Orbital representation diagram depicting each orbital of an atom having its own circle within each sublevel. This diagram is for potassium. Date. 22 February 2010. Source. File:High School Chemistry.pdf, page 355. Author. Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Draw the orbital energy diagram for K (potassium). Represent electrons as arrows (with up or down spin). What is the core] valence electron configuration? (type in the box below) -45 Energy 2p 2s is *** -T-5 ; Question: Draw the orbital energy diagram for K (potassium). Represent electrons as arrows (with up or down spin). Hint :We know that the atomic number of potassium is 19. The electronic configuration of an element describes how electrons are distributed in its atomic ...



















0 Response to "41 orbital diagram for potassium"
Post a Comment