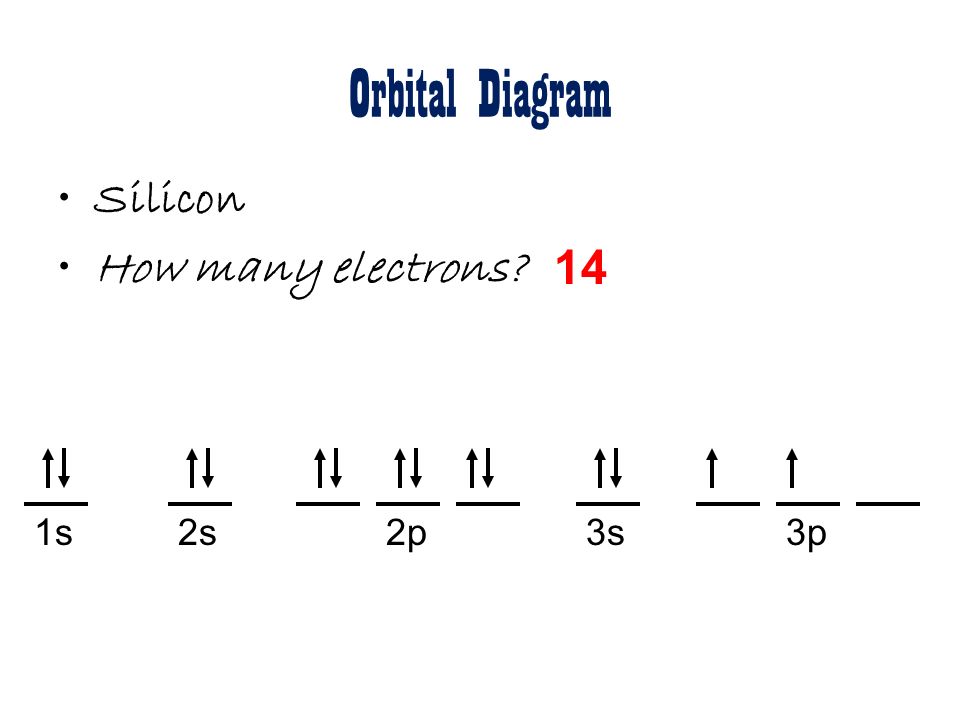
41 orbital diagram for silicon
Silicon Orbital Diagram Orbit diagram consists of a pair of electrons of the atom in the box i.e. Orbit diagram helps to define the ground-state electron configuration is an easy form. That is one box contains 2 electrons. And for silicon, there will be 7 box representations for 14 electrons in a pair. Based on mathematical functions describing the wave-like behavior of either one electron or a pair of electrons in an atom, orbitals can be calculated. Those ...
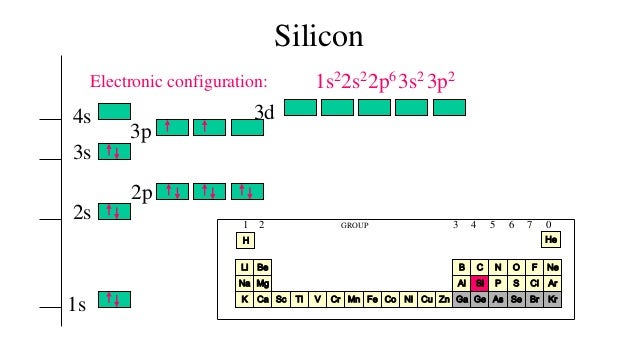
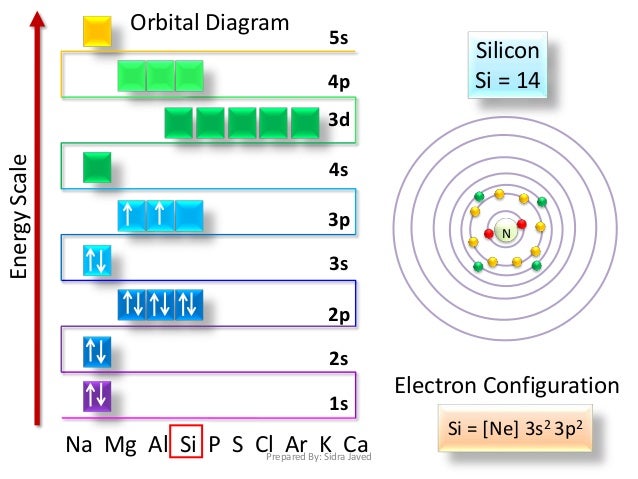
Orbital Diagram, electron configuration, and the noble gas notation for a silicon (Si) atom.
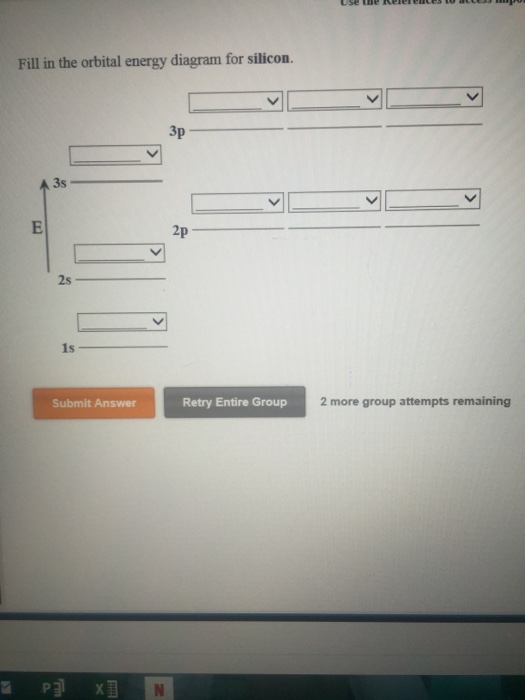
Orbital diagram for silicon
In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. 2 Jun 2018 — 1s^2 2s^2 2p^6 3s^2 3p^2 When adding electrons, the lowest energy levels are always filled first. This is shown by the Aufbau princible ...2 answers · 1s22s22p63s23p2 Explanation: When adding electrons, the lowest energy levels are always ... In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
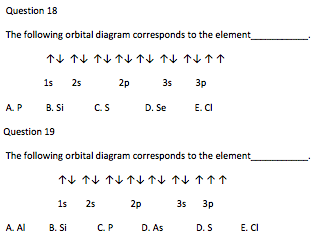
Orbital diagram for silicon. Orbital diagrams must follow 3 rules. The orbitals 1s 2s 2p and 3s are filled first with 2 2 6 and 2 electrons respectively. The Orbital Diagram for Silicon. In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. The nex six electrons will go in the 2p orbital. The Orbital Diagram for Silicon: The orbital diagram for an element shows the electron distribution of the electrons, and the correct pairing of electrons with respect to electron spin. This problem has been solved! Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. plea …. View the full answer. Transcribed image text: Fill in the orbital energy diagram for silicon. Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15 ...
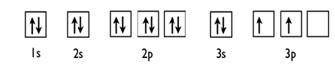
Silicon (Si) ; Oxidation States, +4, -4 ; Electrons Per Shell, 2 8 4 ; Electron Configuration, [Ne] 3s2 3p ; Orbital Diagram. 1s. ↿⇂. 2s. ↿⇂. 2p. ↿⇂. ↿⇂. ↿ ... Orbital diagram for silicon (Si) Silicon (Si) excited state electron configuration Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of silicon is 1s2 2s2 2p6 3s2 3p2. The valency of the element is determined by electron configuration in the excited state. It is a metalloid that has the atomic number 14 in the periodic table. It is in Group 14 of the periodic table. It has the symbol Si. Silicon is the. 8th most abundant element in the universe and is the second most abundant element by weight on earth. It is most commonly found in compounds and never found naturally. In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
2 Jun 2018 — 1s^2 2s^2 2p^6 3s^2 3p^2 When adding electrons, the lowest energy levels are always filled first. This is shown by the Aufbau princible ...2 answers · 1s22s22p63s23p2 Explanation: When adding electrons, the lowest energy levels are always ... In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital.


















0 Response to "41 orbital diagram for silicon"
Post a Comment