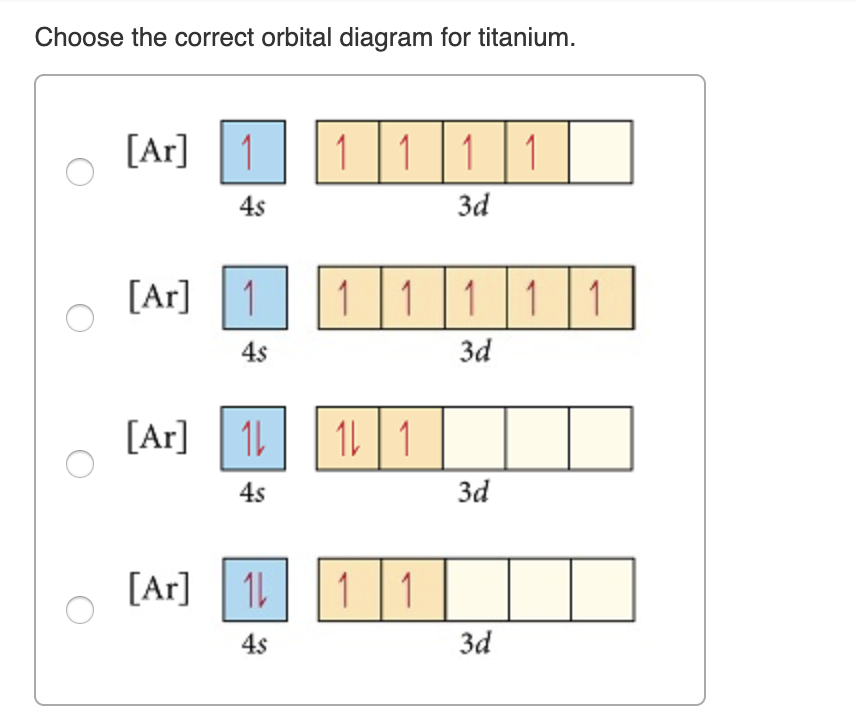
41 orbital diagram for ti
• MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection. What is the orbital diagram for Ti 2+? I got 1s two arrows, 2s two arrows, 2p 6 arrows, 3s two arrows, 3p six arrows, 4s two arrows but it is wrong. It said ions of d-block metals typically lack the outermost s electrons that are present in their neutral counterparts.
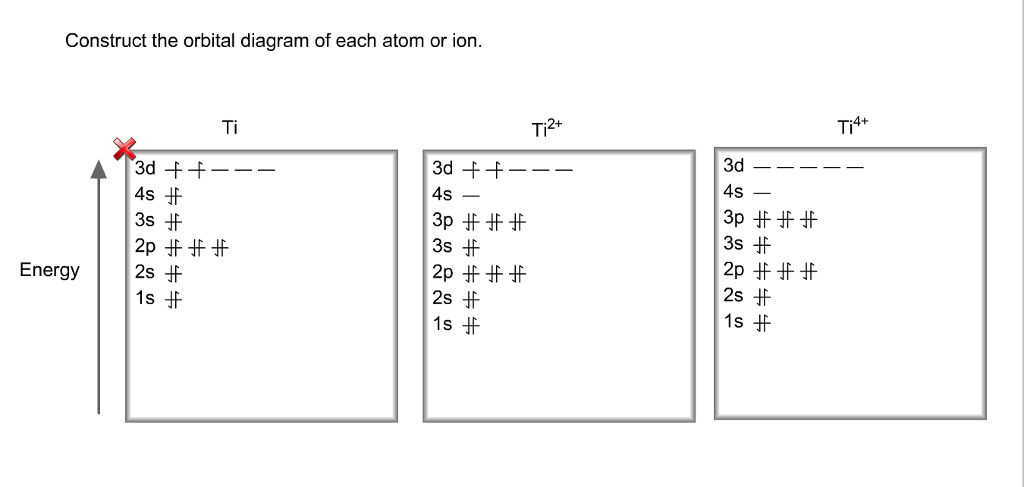
Answer to Construct the orbital diagram of each atom or ion. Ti Ti2+ Ti4+. Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . When filling degenerate orbitals, electrons fill them singly first, with parallel spins is known as.

Orbital diagram for ti
The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells. These energy levels contain sub-shells, or orbitals, each of ... Answer to Solved Identify the orbital diagram of Ti, Ti2+, and Ti4+. To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number...
Orbital diagram for ti. Explain what mistakes have been made in each and draw the correct orbital diagram: Exercise 1s 2s 2p 1s 2s 2p 1s 2s 2p ? ? 1s 2s 2p ? 1s 2s 2p ? 1s 2s 2p ? Draw electrons-in-boxes diagram of the electronic configuration of titanium, Ti (Z = 22). Also, write the ground-state electronic configurations for Ti and Ti 2+ ion. Orbital diagram for ti 4 The Aufbau Principle (also called the building-up principle or the Aufbau rule) allows us to reliably predict the ground state electron configuration of atoms and ions. The Aufbau principle states that, in the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available energy level before ... Draw the orbital diagram for the following elements: Oxygen (O) Titanium (Ti) Silicon (Si) Copper (Cu) For each of the following elements, identify if the electron configuration is correct or incorrect. If it is incorrect, give the fix to the configuration. Carbon (C) = 1s22s22p2. Sulfur (S) = 1s22s22p63p6 Molecular Orbital Study of 02- Adsorbed on Titanium Ions on Oxide Supports ... general energy diagram discussed for coordination modes ... Interaction diagrams for O2 and the Ti(OJ7- fragment for the side-on geometry (to the left of the dashed line) and the end-on ...
Problem: Identify the element which has the following partial orbital diagram a. Ti b. Sn c. Ge d. Zr e. Pr FREE Expert Solution Show answer Answer: Zr. 85% (461 ratings) Sign up for free to keep watching this solution Sign up for free. 575,022. students enrolled. 97%. improved grades ... This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble... Solution for Draw the molecular orbital diagram for [Ti(H2O)6]³+ with the electrons filled in the orbitals. Clearly label the bonding and anti-bonding orbitals. Neon (Ne) electron configuration with full orbital diagram. Neon (Ne) is the tenth element in the periodic table and the 2nd element in group-18. The atomic number of neon is 10 and its symbol is 'Ne'. The standard atomic mass of neon is 20.1797 and it is an inert element.
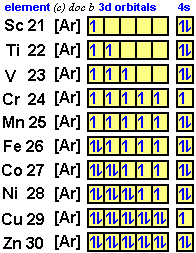
Problem Details. Construct the orbital diagram of each atom or ion. Ti. Ti 2+. Ti 4+. Learn this topic by watching The Electron Configuration: Ions Concept Videos. Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. Molecular orbitals diagrams of [Ti (H2O)6]3+. 1. M. O. diagram for [Ti (H2O)6]3+ Dr. Mithil Fal Desai Shree Mallikarjun and Shri Chetan Manju Desai College Canacona Goa. 2. t* 1u a1g t2g, eg a1g, t1u, eg a1g t1u a* 1g e* g eg t1u Δo t2g Metal (Ti3+)orbitals Ti3+→ [Ar] 3d1, 4s0 1e- Ligand group (H2O) orbitals 6 x 2 = 12 e- σ [Ti (H2O)6]3 ... Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31:
Orbital Diagram. 1s ... 63 Ti: 63: 62.99442(107)# Mass Number The sum of the number of protons and neutrons of an atomic nucleus. In other words, it's the sum of the number of nucleons in an atom. Relative Atomic Mass The ratio of the average mass per atom of an isotope to 1/12 the mass of a carbon-12 atom.
What element does the following orbital diagram represent? Ti. What element has the following electron configuration: 1s²2s²2p⁶3s²3p⁶4s²3d² ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. What is the orbital diagram for Ti 2+?
The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
What is the orbital diagram of each atom or ion? Ti, Ti 2+, Ti 4+ Answer +20. Watch. 1. answer. 0. watching. 726. views. For unlimited access to Homework Help, a Homework+ subscription is required. Christian Garcia Lv10. 26 Jan 2021. Unlock all answers. Get 1 free homework help answer. Unlock. Already have an account? ...
However, once the 4s orbital is filled, it becomes higher in energy than the 3d orbitals. This means that when titanium loses electrons, it does so from the 4s orbital first. Ti: 1s22s22p63s23p63d24s2
Titanium (Ti) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
Construct the orbital diagram of each atom or ion. Ti. Ti 2+ Ti 4+ Next. Practice Problems. Choose the orbital diagram that represents the gro Consider the portion of the orbital filling diagra Choose the orbital diagram that represents the gro Identify the element which has the following parti.
To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number...
Answer to Solved Identify the orbital diagram of Ti, Ti2+, and Ti4+.
The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells. These energy levels contain sub-shells, or orbitals, each of ...



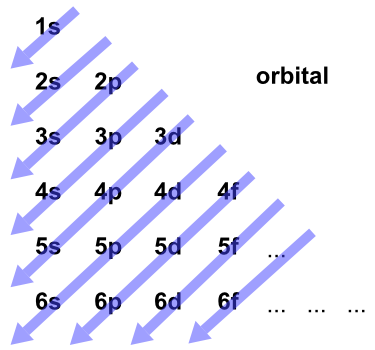

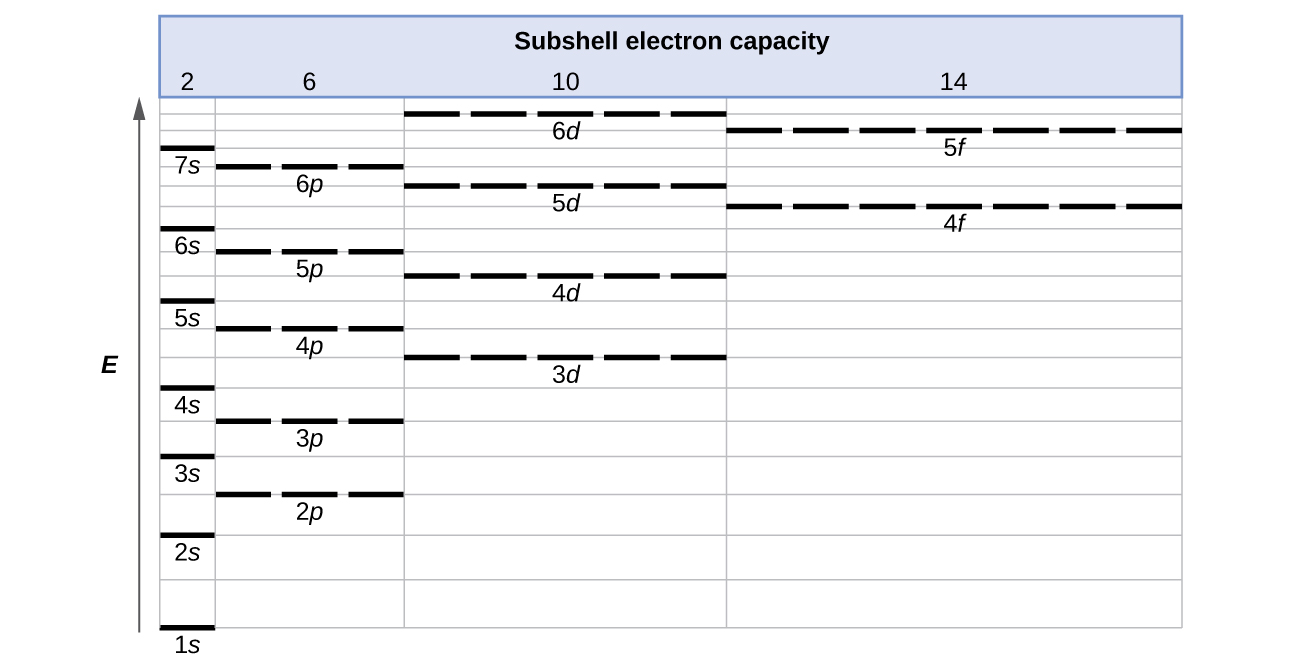























0 Response to "41 orbital diagram for ti"
Post a Comment