42 co2 lewis dot diagram
Draw a dot diagram for boron, showing the number of valence electrons. ... Write Lewis dot diagrams for CO2, NO3 and CO3 as well as indicate the resonance forms if available. How can I draw a Lewis dot diagram for carbon dioxide? Organic Chemistry Lewis Structures and Bonding Lewis Dot Diagram. 1 Answer Deevona Apr 18, 2015 1. 2. Two electrons (dots) make one bond (line). Answer link. Related questions. How is the Lewis structure of an ion written? ...
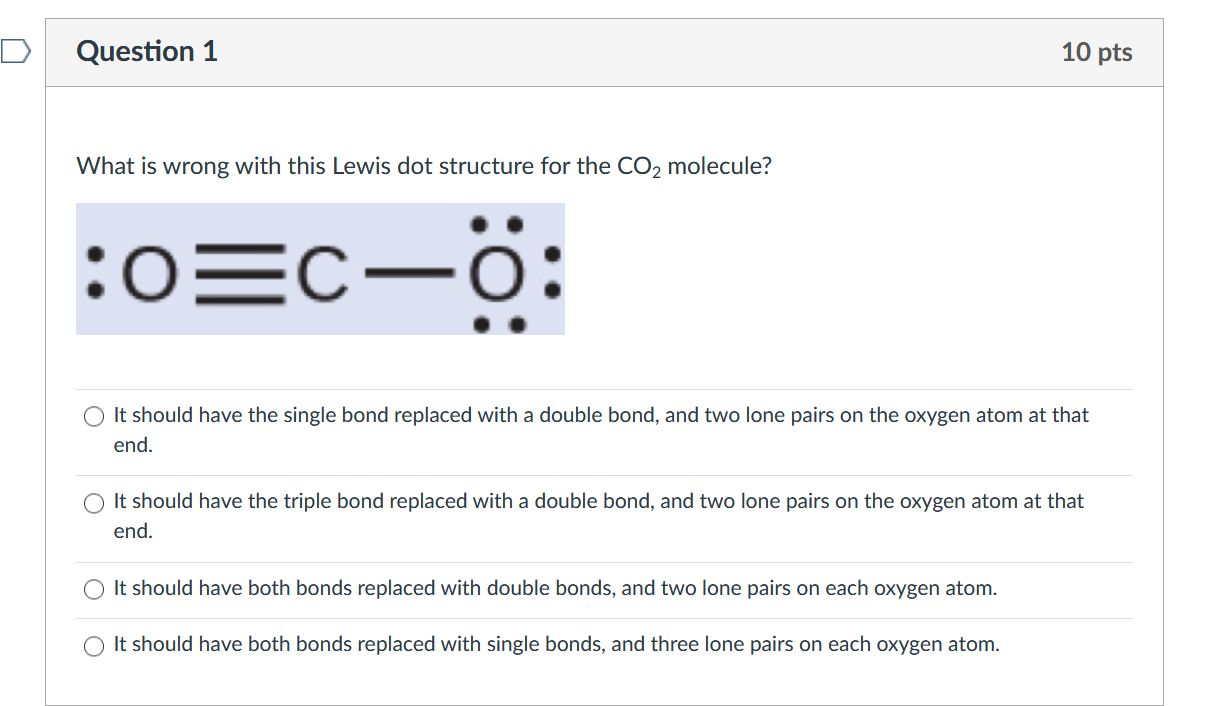
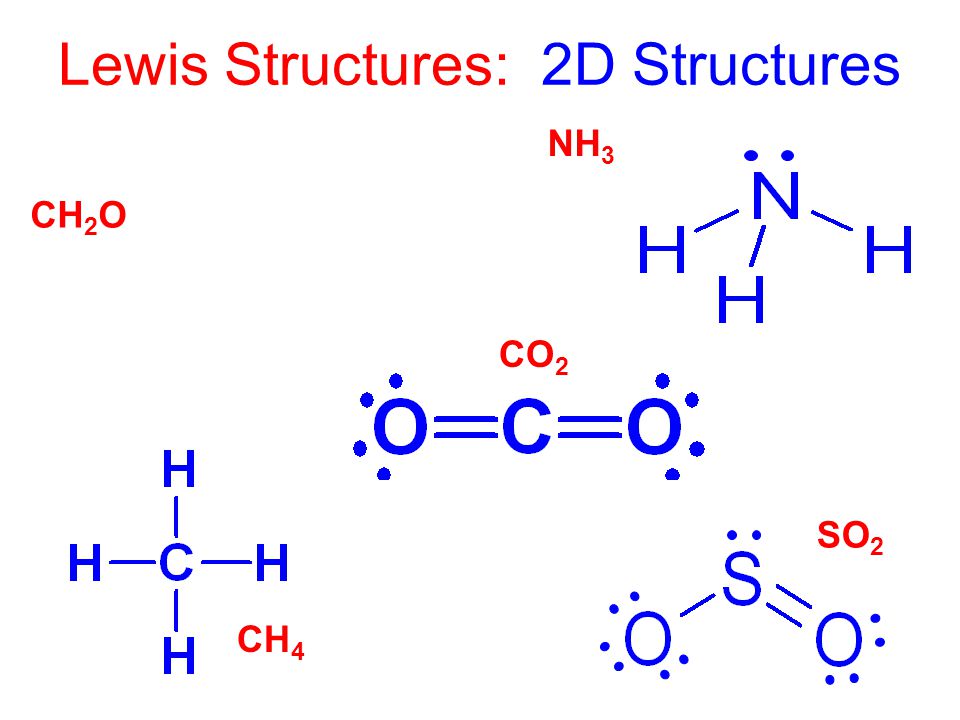
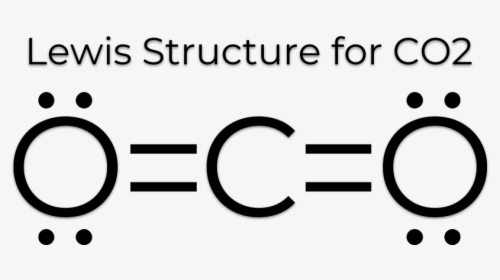
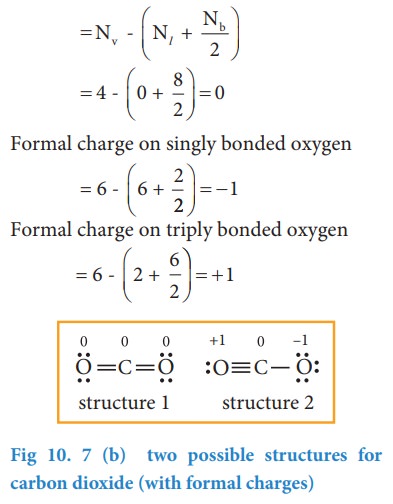
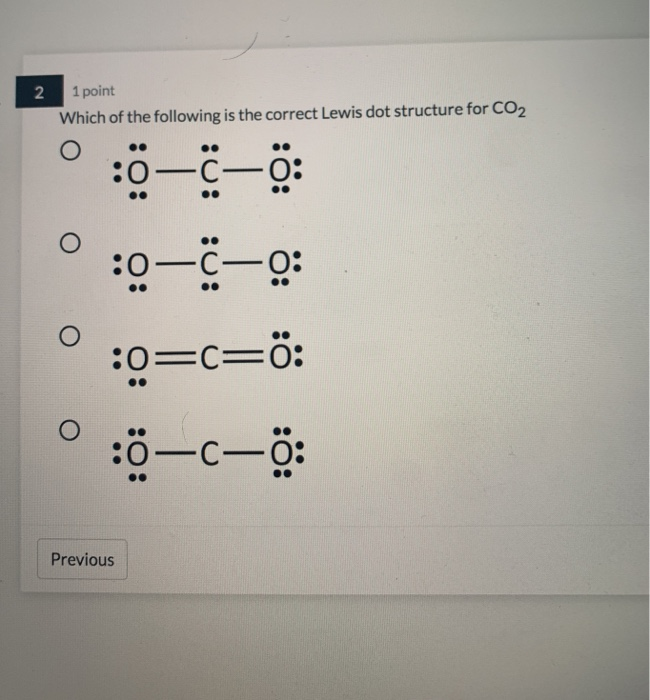
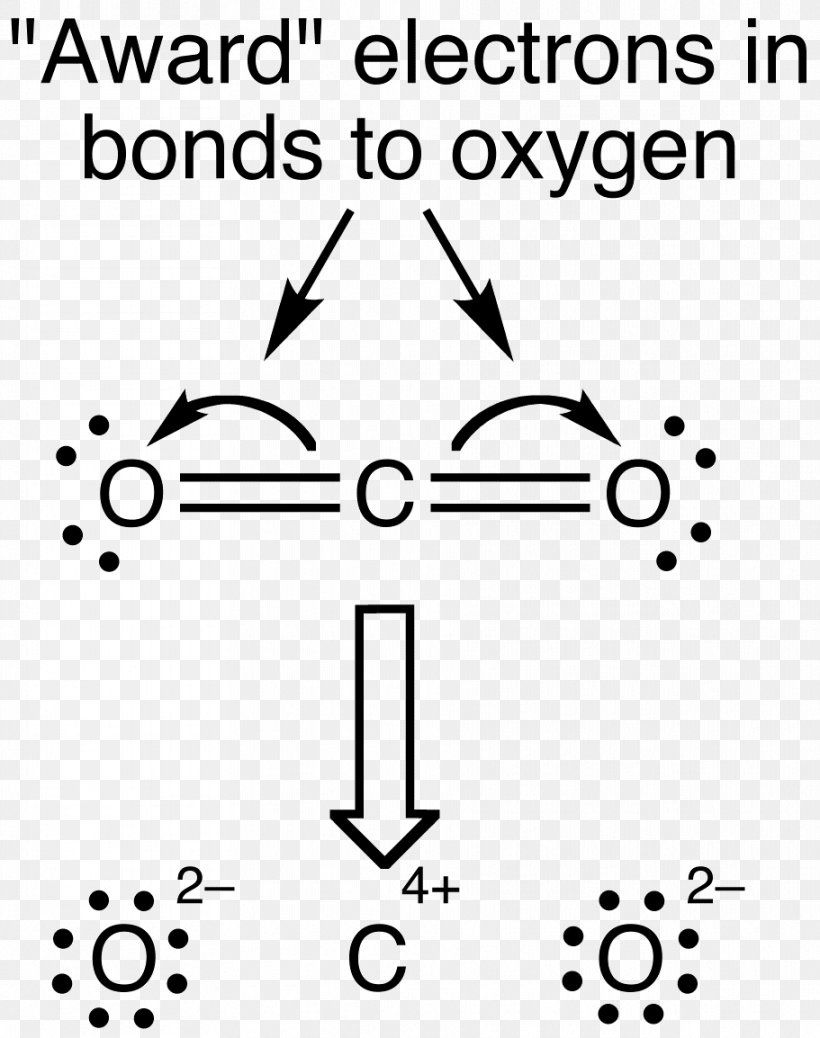
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Co2 lewis dot diagram
CO2 Lewis Properties. The CO 2 Lewis structure is symmetric. Generally, small symmetric molecules are nonpolar. CO 2 is a nonpolar substance, meaning it tends to be a gas. CO 2 has a rather low boiling point of around -80 ℃ or -100 ℉. It can be liquified and even frozen solid with special machinery to produce "dry ice.". Co2 Lewis Structure Shape. Here are a number of highest rated Co2 Lewis Structure Shape pictures upon internet. We identified it from trustworthy source. Its submitted by organization in the best field. We take this kind of Co2 Lewis Structure Shape graphic could possibly be the most trending subject behind we part it in google plus or facebook. 1 päivä sitten · Below, we have a diagram explaining the MO of N2H2. Conclusion. In this article, we have discussed the chemical bonding inside a diazene molecule. We have explained in detail the Lewis Structure, molecular geometry, hybridization, and MO diagram for the N2H2 molecule. Happy learning!
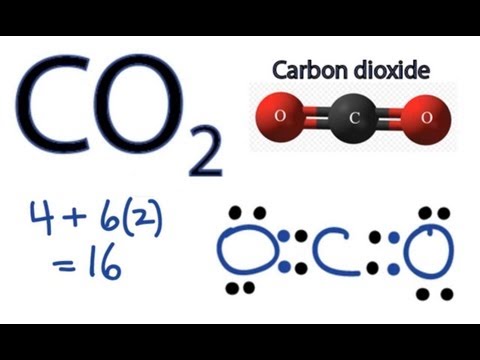
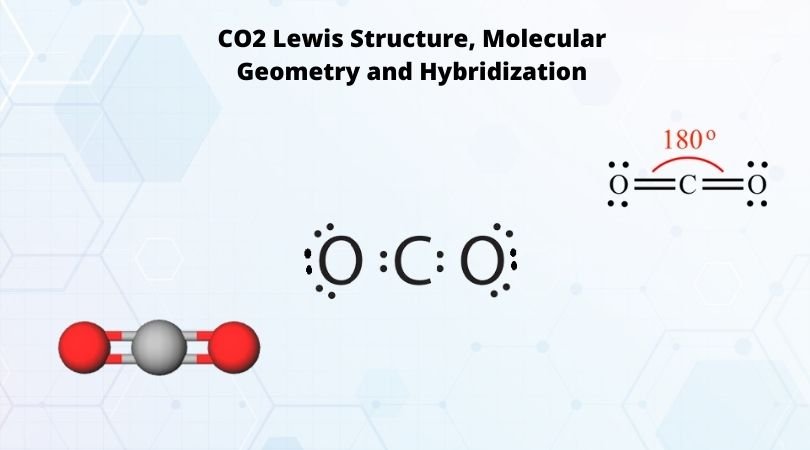
Co2 lewis dot diagram. Carbon dioxide (CO2) has a total of 16 valence electrons which present on the outer shell of atoms i.e. 4 carbon atoms and 12 of two oxygen atoms. From this we can easily draw the Lewis dot diagram of CO2 by adjusting two double bonds between carbon and oxygen (O=C=O). A step-by-step explanation of how to draw the CO2 Lewis Dot Structure (Carbon dioxide).For the CO2 structure use the periodic table to find the total number ... Carbon dioxide (CO 2) lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO 2. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO 2 is linear. Steps of drawing the lewis structure of CO 2 are explained in detail in this tutorial. CO2 can be used to flood the surgical field during cardiac surgery. Because of its density, carbon dioxide displaces the air surrounding the open heart so that any gas bubbles trapped in the heart are carbon dioxide rather than insoluble nitrogen.Similarly, CO2 is used to de-bubble cardiopulmonary bypass and extracorporeal membrane oxygenation (ECMO) circuits.
To know the lewis structure of CO2, one should first understand what precisely the Lewis structure is. Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule. These valence electrons are represented by drawing dots around the individual atoms, hence the Lewis dot structure. Lewis Structure of Carbon Dioxide ( C O 2). In ( C O 2), oxygen belongs to group 16 of the Periodic Table, and carbon belongs to group 14 of the Periodic Table. Hence, oxygen has 6 valence electrons, and carbon has 4 valence electrons. Step 1- In ( C O 2) we, have C = 4 × 1 = 4 valence electrons. O = 6 × 2 = 12 valence electrons. What is the Lewis dot diagram for carbon? Only the electrons in the valence level are shown using this notation. For example, the Lewis symbol of carbon depicts a "C' surrounded by 4 valence electrons because carbon has an electron configuration of 1s22s22p2. The Lewis symbol for carbon: Each of the four valence electrons is represented as a dot. Methyl propionate | C2H5COOCH3 or C4H8O2 | CID 11124 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological ...
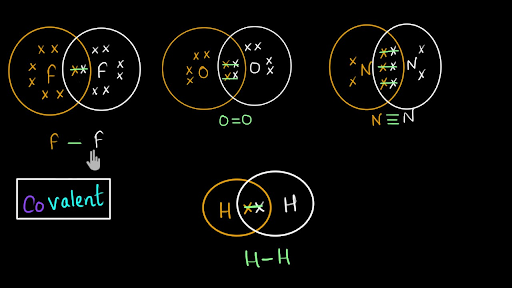
Electron dot structures or Lewis dot formula - It defines the nature of bond and position of atoms of the molecule which are connected in the molecule. Electron dot structures of carbon dioxide. The carbon is the central atom of this molecule. Oxygen atom contains 6 valence electrons which form 2 lone pairs. I quickly take you through how to draw the Lewis Structure of CO2 (Carbon DiOxide). I also go over hybridization, shape and bond angles. It is represented by dots in the CO2 Lewis diagram. The CO2 molecule's core carbon atom can be represented as follows: Total outermost valence shell electron of oxygen atom in CO2= 6 Total outermost valence shell electron of carbon atom in CO2= 4 The CO2 molecule has one central carbon and two oxygen atoms. 21.11.2021 · Review what a Lewis dot diagram is and discover how to draw a Lewis dot structural formula for compounds. Learn how to represent single, double and triple bonds with lines instead of dots.
Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure work on the octet rule, which means that all the atoms in the structure would have eight electrons in their valence shell except hydrogen.
Lewis structure of co2 for counting valence electrons around the terminal oxygen atoms. To draw the co2 lewis structure, we have to find out the co2 valence electrons first.we express valence electrons as dots in co2 lewis dot structure. Lewis structure of carbonate ion is drawn in this tutorial step by step.
The Lewis Dot Structure for CO2. Created by MakeTheBrainHappy. This is the Lewis Dot Structure for CO2. You could alternatively also draw the structure by including two dots for every bond. That would mean that you would have a total of eight dots around the carbon, thereby filling its octet. The octets of both of the oxygen atoms are also ...
A Lewis Structure or Lewis Dot Structure is a formula developed to concretely express the bond formation between chemical species (atom, molecule, ion). Lewis Structure shows the electrons in the last layer of the atom with dots around the symbol of the atom. With this way; the number of electrons in the last layer of the atom, the desire to ...
The Lewis Dot Structure is a graphical representation of how electrons are distributed around the atoms which comprise a molecule. The reason for drawing/creating a Lewis dot structure is that it helps one predict the kinds of bonds, as well as a number of bonds, that can be formed around an atom. Lewis structures can be utilized to make ...
5.3: Lewis Diagrams. What is the Lewis symbol for Na+? Na becomes Na+ by losing one electron from its valence shell and getting an overall positive charge. Hence, the Lewis symbol for the sodium ion does not have a dot, but a plus sign as a superscript. What is the formula for Na2CO3? Na₂CO₃ Carbonato de sódio/Fórmula. Is baking soda ...
Draw Lewis dot diagram for the following. Carbon dioxide (CO2) Maharashtra State Board HSC Science (General) 11th. Textbook Solutions 8028 Important Solutions 18 ... Draw Lewis dot diagram for the following. Carbon dioxide (CO 2) Advertisement Remove all ads. Solution Show Solution.
CO2 lewis structure contains two oxygen atoms and one carbon atom, connected with the double bond whereas carbon is the central atom, and no lone pair is present on it. But each oxygen in the CO2 lewis dot structure has two lone pairs. A lewis diagram helps us to know how electrons are arranged around individual atoms in a molecule.
CO2 Lewis structure So CO2 = 4 + 6 (2) = 16. So, total valence electrons are 16. Carbon is the least electronegative that means it stays at the center. So, put the Carbon in the middle and then set the oxygen either side of that! Additionally, how many lone electron pairs are there in the Lewis dot formula for carbon dioxide? in carbon dioxide ...
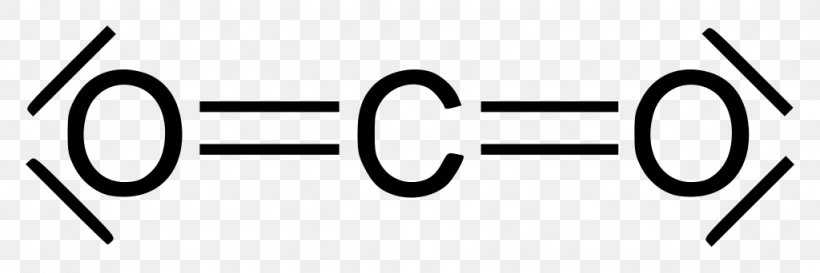
Lewis Dot of Carbon Dioxide. CO 2. Back. 70 More Lewis Dot Structures. Produced from the complete combustion of hydrocarbons. Used by plants during photosynthesis. CO 2 contains 2 double bonded oxygen atoms 180 o apart. This symmetrical structure gives it a nonpolar shape and weak intermolecular forces. YouTube.
25.2.2020 · SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ].
What is the electron dot diagram of carbon dioxide? The molecular formula of carbon dioxide is CO2. - The Lewis dot diagram is also called the Lewis electron dot structure, here the valence electrons present in the compound are represented as dots around the atoms. Why is carbon dioxide a Lewis acid?
Lewis dot structure for Cl2 (Chlorine gas) By looking at the above Cl2 lewis structure, we see both chlorine atoms completed their octet comfortably as both of them have 8 electrons around them. And no need to make any covalent bond in this lewis diagram because we got our stable lewis dot structure for Cl2.
Lewis dot structure of co2 molecule Lewis symbols use dots to visually represent the valence electrons of an atom. Recall the Lewis structure formalism for representing valance electrons Key Takeaways Key Points Electrons exist outside of an atom 's nucleus and are found in principal energy levels that contain only up to a specific number of electrons.
6.5.2018 · Dispersion Forces CO_2 has dispersion forces or van der waals forces as its only intermolecular force. Since CO_2 is made of one carbon and 2 oxygen and both carbon and oxygen are non-metals, it also have covalent bonds. For extra information, there are 3 types of intermolecular forces. Dispersion Forces Dipole-dipole Hydrogen bonds Dispersion forces …
CO2 Lewis Structure. The lewis structure of CO2 can be with some simple steps, but before that, it is important to understand lewis structure properly. So lewis structure generally gives us an idea about the nature of bonding and octet fulfillment of the atoms. According to the octet rule, an atom attains stability by fulfilling its octet.
6 Steps on How to Draw CO2's Lewis Structure Calculate the total valence electrons found in a molecule. Carbon Valence Electron=4 Oxygen Valence electrons: 6*2 = 12 Total number of valence electrons = 16 Find the central atom, which is usually the one with the highest bonding sites, is the Carbon atom. Draw four dots around it.
2 päivää sitten · Molecular Geometry and MO Diagram of SF6 There are Fluorine atoms all around Sulfur which gives the compound a kind of symmetry when we look at it on a planar level. When we look at the molecular geometry of any compound we get to see a 3-D image of how the atoms are distributed which we cannot identify while making a Lewis Structure.
1 päivä sitten · Below, we have a diagram explaining the MO of N2H2. Conclusion. In this article, we have discussed the chemical bonding inside a diazene molecule. We have explained in detail the Lewis Structure, molecular geometry, hybridization, and MO diagram for the N2H2 molecule. Happy learning!
Co2 Lewis Structure Shape. Here are a number of highest rated Co2 Lewis Structure Shape pictures upon internet. We identified it from trustworthy source. Its submitted by organization in the best field. We take this kind of Co2 Lewis Structure Shape graphic could possibly be the most trending subject behind we part it in google plus or facebook.
CO2 Lewis Properties. The CO 2 Lewis structure is symmetric. Generally, small symmetric molecules are nonpolar. CO 2 is a nonpolar substance, meaning it tends to be a gas. CO 2 has a rather low boiling point of around -80 ℃ or -100 ℉. It can be liquified and even frozen solid with special machinery to produce "dry ice.".









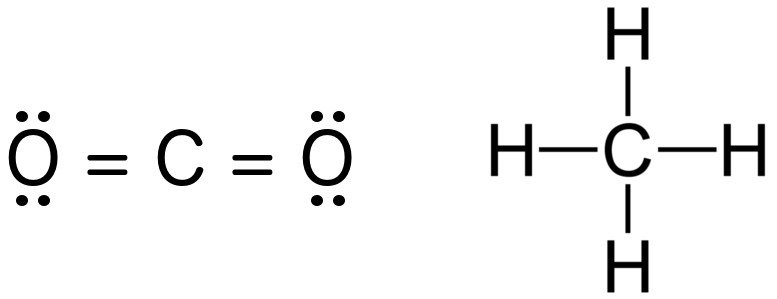



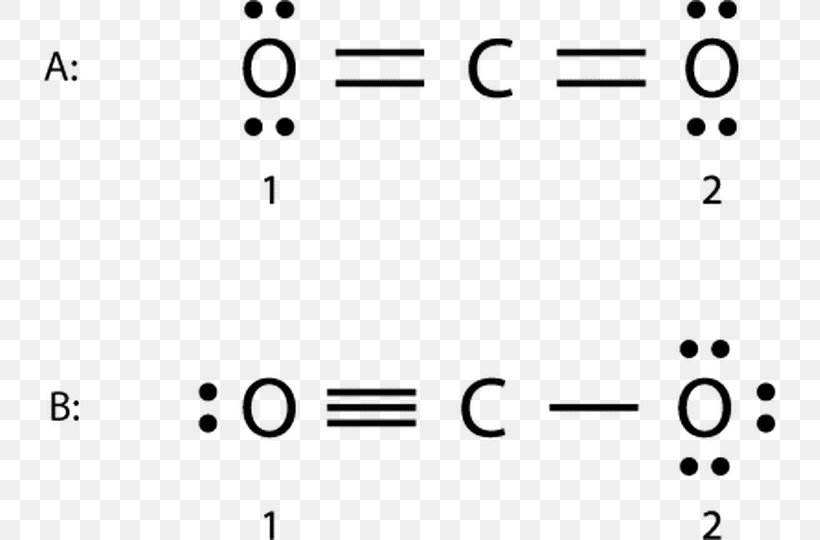












0 Response to "42 co2 lewis dot diagram"
Post a Comment