40 Endothermic Reaction Coordinate Diagram
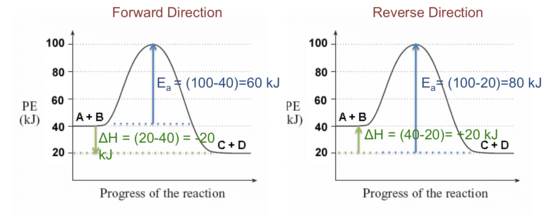
Reaction Coordinate Diagrams - University of Illinois Urbana ... Reaction Coordinate Diagram of Ozone Photolysis The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction . Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction. PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... In this reaction, the total energy of the reactants is 80 kJ mol-1, the total energy of the products is -90 kJmol-1 and the activation energy for the forward reaction is 120 kJ mol-1. a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic.
Thermodynamics, reaction coordinate diagram, Gibbs free-energy ... Make 12 monthly payments of $274.58 | Pay 0% interest | Start using the domain today. See details · Call us to learn how we can help you get more value from your domain

Endothermic reaction coordinate diagram
Exothermic and Endothermic Processes | Introduction to Chemistry Endothermic and exothermic reactionsPaul Andersen explains how heat can be absorbed in endothermic or released in exothermic reactions. An energy diagram can be used to show energy movements in these reactions and temperature can be used to measure them macroscopically. The Reaction Coordinate Diagram - YouTube The energy changes that accompany each step of a reaction mechanism are represented on a reaction coordinate diagram. The reaction pathway on this plot is t... PDF Reactions of Alkenes - University of Texas at Austin • Energy diagram: A graph showing the changes in energy that occur during a chemical reaction. • Reaction coordinate: A measure in the change in positions of atoms during a reaction. Reaction coordinate Energy Energy Diagrams 6 • Transition state ‡: - An unstable species of maximum energy formed during the course of a reaction.
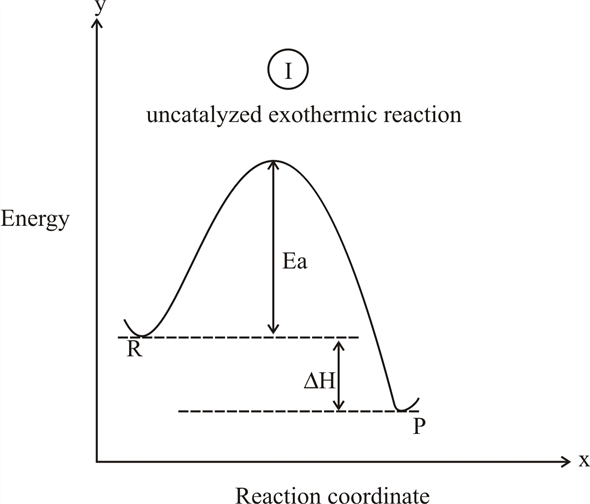
Endothermic reaction coordinate diagram. Reaction Coordinate Diagram Endothermic Vs Exothermic A reaction will be exothermic if the energy of the products is less than the energy of the Below is a reaction coordinate diagram for an endothermic reaction. A general Reaction Coordinate Diagram relating the energy of a system to leading to an exothermic reaction (∆H 0). Endothermic Reaction. PDF EnergyDiagrams - Towson University • Energy Diagrams are a plot of the reacon steps,&or"ReaconCoordinate"(Xaxis)versus theEnergy(KcalorKJ) ENERGY REACTION COORDINATE • Ina spontaneous&reacon,&theproduct(s)are more stable than the reactant(s),& thus the ... REACTION COORDINATE NON-SPONTANEOUS OR ENDOTHERMIC Analyzing Energy With a Reaction Coordinate Diagram And a reaction coordinate diagram where the energy level of B ends up lower than A is exothermic (delta E is negative): Activation Energy The activation energy is important in a reaction. Even if... Reaction Kinetics: Reaction Mechanisms: Mechanisms of Chemical ... A reaction will be exothermic if the energy of the products is less than the energy of the reactants. A reaction is endothermic when the energy of the products is greater than the energy of the reactants. The is for an exothermic reaction. Below is a reaction coordinate diagram for an endothermic ...
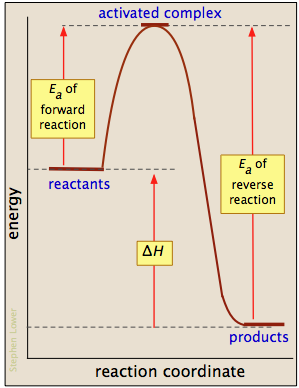
6.3.2: Basics of Reaction Profiles - Chemistry LibreTexts August 15, 2020 - In examining such diagrams, take special note of the following: The "reaction coordinate" plotted along the abscissa represents the changes in atomic coordinates as the system progresses from reactants to products. In the very simplest elementary reactions it might correspond to the stretching ... HOW TO: Draw and interpret reaction coordinate Energy plots Reaction coordinate plots are very useful in showing: 1) whether a reaction is an exo- or endothermic process, 2) which reaction pathway is faster, and 3) which products are the kinetic (fastest formed) and thermodynamic (most stable) products. Bridget Thomson - PE Diagram Sheet.pdf - POTENTIAL ENERGY ... View Bridget Thomson - PE Diagram Sheet.pdf from CHE 1 at Scarsdale Highschool. POTENTIAL ENERGY DIAGRAM Name Potential Energy C+D Reaction Coordinate C + D + energy Answer the questions using the PDF Thermodynamics vs Kinetics - Columbia University A general Reaction Coordinate Diagram relating the energy of a system to its geometry along one possible reaction pathway is given in the figure below. In the figure below, the Activation Energy, Ea is that critical minimum energy in a chemical reaction required by reactants to be converted into products. the quantities, Ea;
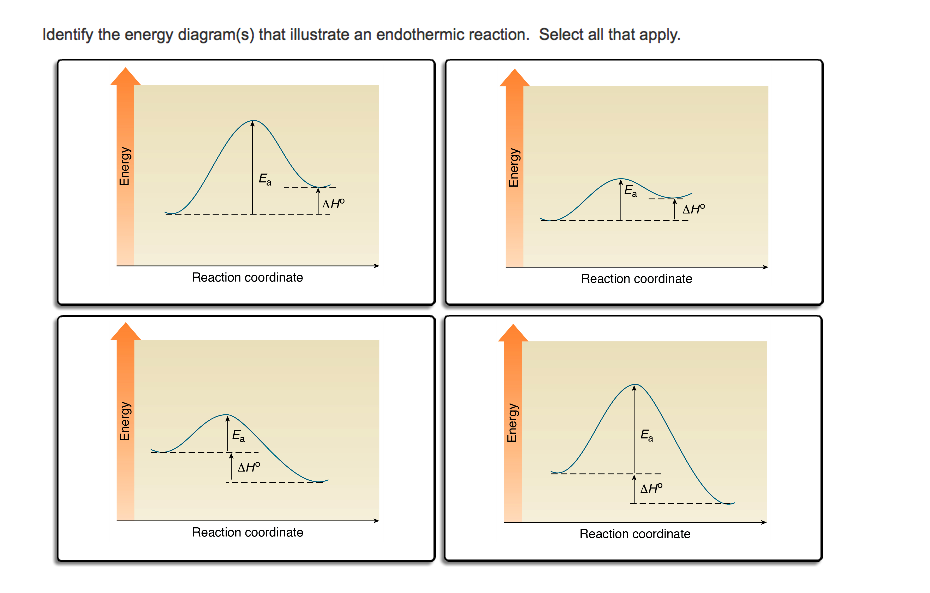
Solved Which of the following energy diagrams shows a ... Which of the following energy diagrams shows a concerted endothermic reaction? free energy. kJ/mol Toitwool adolorib919 reaction coordinate reaction coordinate reaction coordinate reaction coordinate yam 2692 iw noite91 i r tobas (16.101 DA to ngiz adib919 Endothermic Reaction Coordinate Diagram - schematron.org Sep 16, 2018 · The " reaction coordinate " plotted along the abscissa represents the diagrams can describe both exothermic and endothermic reactions. A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. The fully filled in reaction coordinate diagram is displayed below. Arrhenius Theory and Reaction Coordinates Typically, we envision reactions proceeding left to right along the reaction coordinate, so often, the activation energy is only noted for the forward reaction. The activation energy on the diagram below shows the barrier to be 102.6 kJ mol -1 . Reaction Coordinate Diagram Endothermic - Wiring Diagrams A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. The " reaction coordinate " plotted along the abscissa represents the diagrams can describe both exothermic and endothermic reactions.A reaction will be exothermic if the energy of the products is less than the energy of the reactants.
6.6: Reaction Coordinate Diagrams - Chemistry LibreTexts In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical one-step reaction might look like this:
Endothermic Reaction Coordinate Diagram - Wiring Diagrams A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other. Let's consider a general reaction where a reactant or set of reactants, A, or set of products, B. The diagram below is called a reaction coordinate diagram. B is at a lower total ...
An endothermic reaction with high activation energy for ... Solution Verified by Toppr Correct option is C) In an endothermic reaction, the overall energy is absorbed or taken in by the system and therefore,the energy of the product is higher than that of the reactant. An endotheremic reaction with high activation energy for the forward reaction is given by the diagram C.
Reaction Coordinate Diagrams - College Chemistry The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants.
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
5.3. Reaction coordinate diagrams | Organic Chemistry 1: An open ... In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ‘reaction coordinate’, tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical SN2 reaction ...
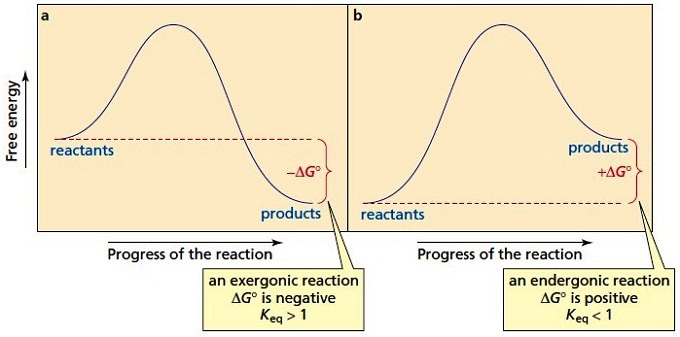
Energy profile (chemistry) - Wikipedia The ∆G° can be written as a ... 100 °C). A reaction with ∆H°<0 is called exothermic reaction while one with ∆H°>0 is endothermic. Figure 8: Reaction Coordinate Diagrams showing favorable or unfavorable and slow or fast reactions...
18.4: Potential Energy Diagrams - Chemistry LibreTexts 1 week ago - The energy changes that occur during ... called a reaction progress curve. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an ...
PDF NAME Kinetics Potential Energy Diagrams - ISD 622 Which labeled section on the diagram for the endothermic reaction shows the most significant difference between the diagrams for the exothermic vs. ... Reaction Coordinate X + Y + energy XY (continued) 7. On the diagram below, draw a dotted line to show the pathway of a catalyzed reaction. Add the
reaction coordinate diagrams The energy to reach the transition state, whether the reaction is endo- or exothermic, comes from collisions between molecules · All molecules possess kinetic energy at temperatures above 0 K, which is distributed among them according to a Boltzman distribution · Raising the temperature raises ...
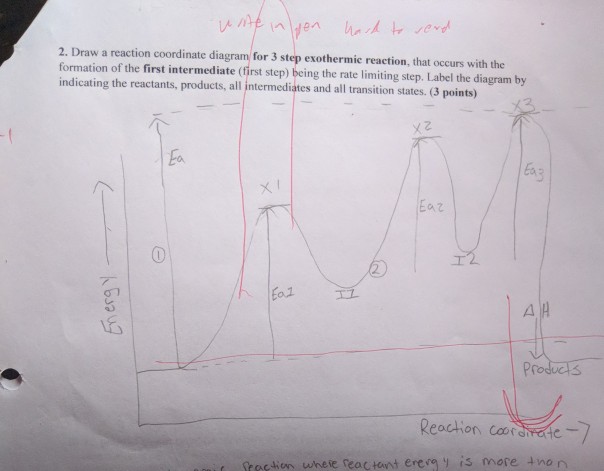
04.02 Reaction Coordinate Diagrams and Stability Trends ... General structure of a reaction coordinate diagram, including transition states and intermediates. Overall free energy change and activation energy. Definiti...
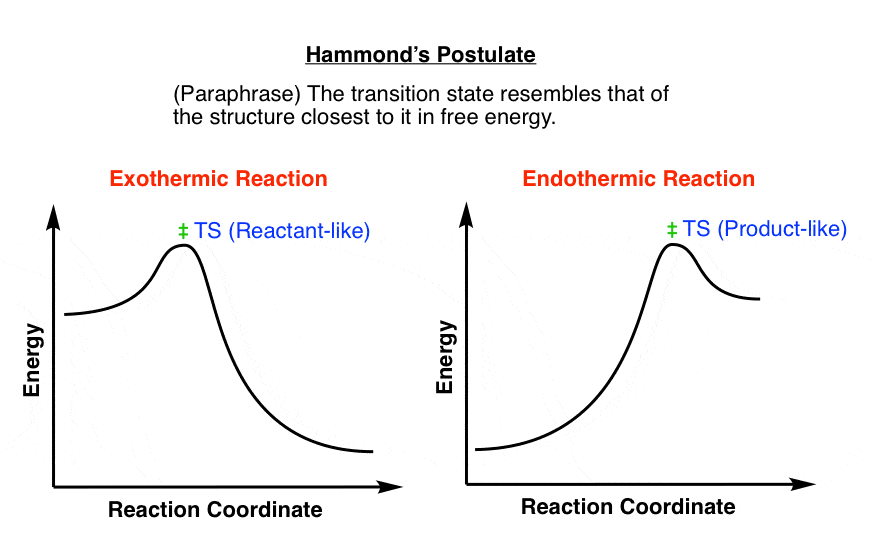
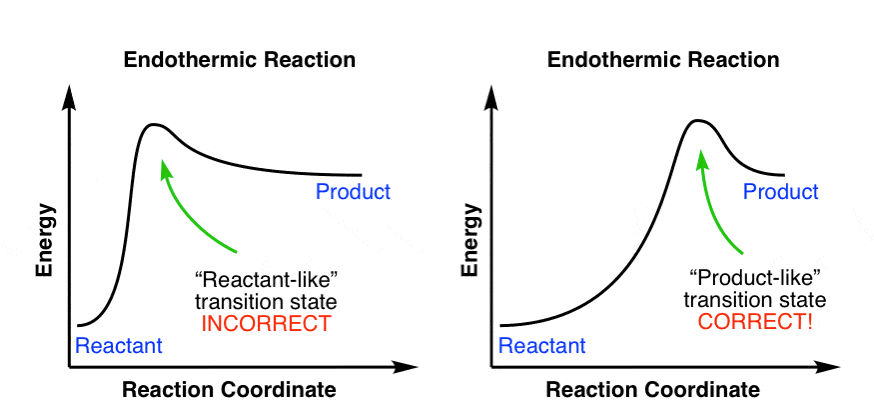
6.8: Kinetics - Chemistry LibreTexts August 11, 2020 - In the exothermic diagram on the left, a significant activation energy must be provided to initiate the reaction. Since the reaction is strongly exothermic, it will probably generate enough heat to keep going as long as reactants remain. The endothermic reaction in the center has a similar ...
Reaction Coordinate Diagrams It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction ( ...
Endothermic vs. exothermic reactions (article) | Khan Academy Energy diagrams for endothermic and exothermic reactions In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, the products are less stable than the reactants.
What are Endothermic Reactions? (with Examples & Video) Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react.
6.9: Describing a Reaction - Energy Diagrams and ... In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical one-step reaction might look like this:
Illustrated Glossary of Organic Chemistry - Endothermic Energy profile for a concerted endothermic reaction. Related terms: Endergonic, exergonic, exothermic, ΔG, ΔG++, activation energy, energy of activation, Eact, enthalpy of formation, heat of formation, enthalpy of hydrogenation, heat of hydrogenation, enthalpy of combustion, heat of combustion, equilibrium, reaction coordinate...
PDF imarkic.weebly.com Construct a reaction coordinate diagram (as in Figure 1) for a typical endothermic reaction. Which has the larger activation energy, the forward reaction or the reverse reaction ? 02(g) + N02(g) has 10.7 and The reaction 03(g) + NO(g) AH = —199.8 kJ/mole. What is the activation energy for the following reaction?
Endothermic reaction diagram - ALEKS Jennifer Kwong 8:50 ... Chemists often use reaction energy diagrams to understand how chemical potential energy changes during a chemical reaction There are three important facts about a reaction that its energy diagram can tell you:. A. The heat of reaction.
PDF Energy/Reaction Coordinate Diagrams 1! Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay" A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction
chemical energetics - an introduction Basic ideas about energy changes during chemical reactions, including simple energy diagrams and the terms exothermic and endothermic.
The diagram represents a spontaneous react... | Clutch Prep Is the reaction endothermic or exothermic? What is the activation energy of the reaction? FREE Expert Solution Recall that an energy diagram is usually read from left to right. ... Q. Consider the Potential Energy vs. Reaction Coordinate diagram shown to the right. For each of the following questions, circle the best answer.If a...
Energy Diagram — Overview & Parts - Expii Energy diagrams are also referred to as reaction coordinate diagrams! ... Endothermic reactions gain energy/heat so when drawing the energy diagram, ...
PDF Garden City Public Schools / Homepage Reaction Coordinate Does this potential energy diagram represent an exothermic or an endothermic reaction? [Explain why.] According to the diagram, is the po 'al energy of the products greater than, less than, or equal to the potential energy of the reactants?
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In endothermic reactions, there is less energy in the reactants than ...
Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction
PDF Reactions of Alkenes - University of Texas at Austin • Energy diagram: A graph showing the changes in energy that occur during a chemical reaction. • Reaction coordinate: A measure in the change in positions of atoms during a reaction. Reaction coordinate Energy Energy Diagrams 6 • Transition state ‡: - An unstable species of maximum energy formed during the course of a reaction.

Which reaction coordinate diagram represents a reaction in which the activation energy, Ea, is 50 kJ•mol –1 and the ΔHrxn is –15 kJ•mol –1?
The Reaction Coordinate Diagram - YouTube The energy changes that accompany each step of a reaction mechanism are represented on a reaction coordinate diagram. The reaction pathway on this plot is t...
Exothermic and Endothermic Processes | Introduction to Chemistry Endothermic and exothermic reactionsPaul Andersen explains how heat can be absorbed in endothermic or released in exothermic reactions. An energy diagram can be used to show energy movements in these reactions and temperature can be used to measure them macroscopically.






















0 Response to "40 Endothermic Reaction Coordinate Diagram"
Post a Comment