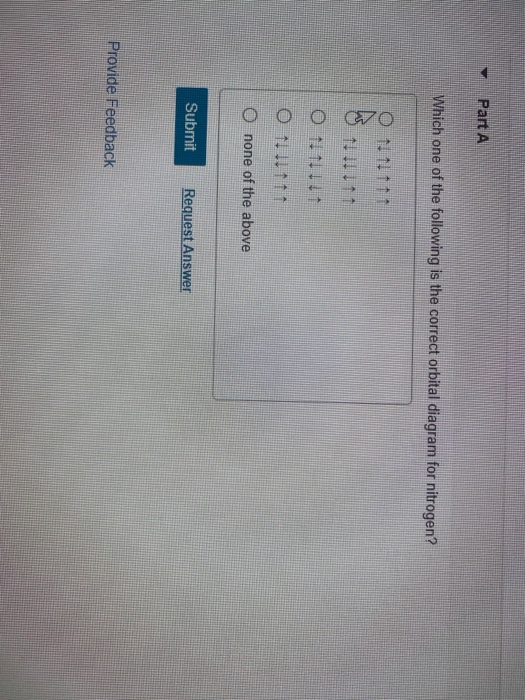
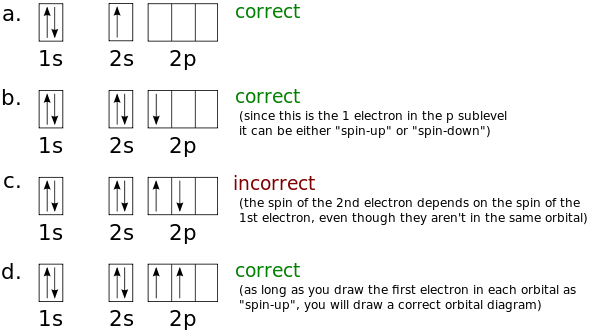
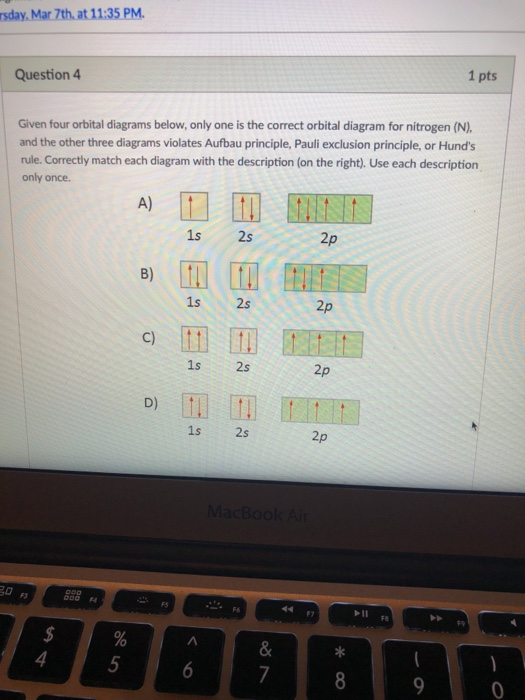
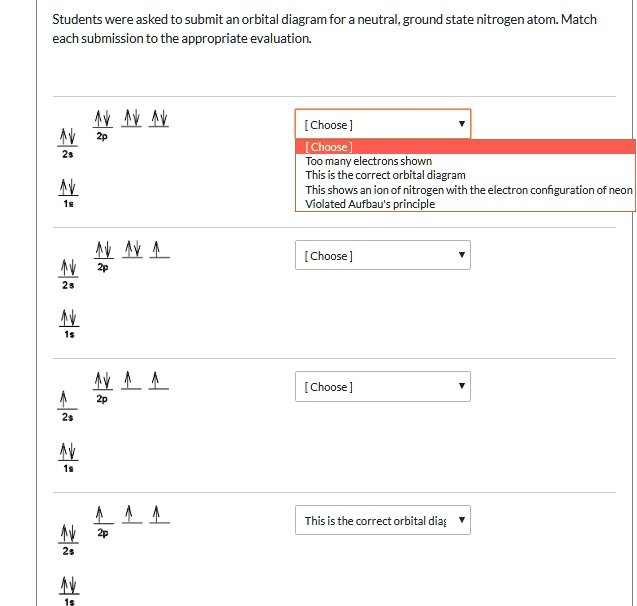
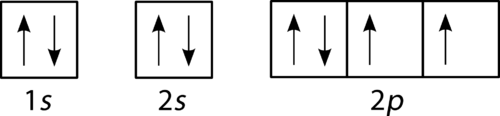
41 which one of the following is the correct orbital diagram for nitrogen?
Solved Which one of the following is the correct orbital | Chegg.com. Science. Chemistry. Chemistry questions and answers. Which one of the following is the correct orbital diagram for nitrogen? Select one: O a. 1 1 1 1 O b. 11 11 1 1 1 Oct 11 7 7 O d. 11 11 7 7 7. Question: Which one of the following is the correct orbital diagram for nitrogen ... Hund's rule states that every orbital is a subshell is singly occupied with one electron before any one orbital is doubly occupied and are an electron in singly occupied orbitals should have the same spin.
1.1.6: Which of the following is a chemical change? Explain your reasoning... ... 1.1.46: Each of the beakers depicted below contains two liquids ...
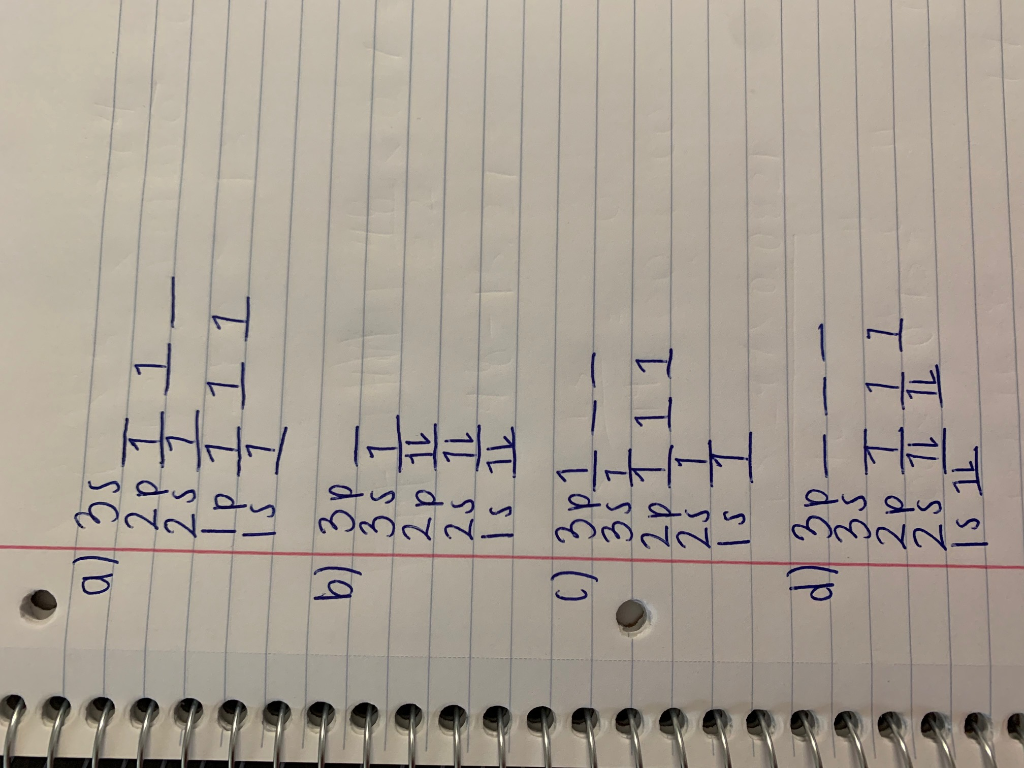
Which one of the following is the correct orbital diagram for nitrogen?
07.06.2018 · For example in the following dipeptide we have two different protecting groups on nitrogen – one Boc and one CBz. By selecting “orthogonal” protecting groups, each nitrogen is addressable – we can choose which protecting group to remove, and our synthesis can proceed from there. This avoids getting into a situation where we have two unprotected amines and … Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s. Each gene in italics in the diagram produces one enzyme necessary for the synthesis of this essential amino acid required for growth. Table 1: Growth response of mutant strains in "minimal" media with supplements as indicated. Growth is indicated by (+), and no growth is indicated by (-). Question 2. According to the information provided, a conclusion that can be made with …
Which one of the following is the correct orbital diagram for nitrogen?. Above orbital diagram shows the electron configuration of nitrogen atom. ... Correct option is . B. Hund's rule. Hund's rule states that every orbital is a subshell is singly occupied with one electron before any one orbital is doubly occupied and are an electron in singly occupied orbitals should have the same spin. ... Consider the following ... Which one of the following is the correct orbital diagram for nitrogen? Q. Part A. In the animation, you can see that the electrons occupy different orbitals according to the energy level of each orbital. The water immersion program involved a series of tests in which the H-1 engine was immersed in sea water for given periods of time, followed by ... ... of 178 Q&A communities including Stack Overflow , the largest, most trusted online community for developers to learn, share their knowledge, and ...
Which one of the following is the correct orbital diagram for nitrogen? Learn this topic by watching The Electron Configuration Concept Videos All Chemistry Practice Problems The Electron Configuration Practice Problems Which one of the following is the correct orbital diagram for nitrogen? Consider the electron configuration of the ion to determine which ion shown below has an incorrect ionic charge? Al3- ... 18 minutes ago If you do not know the densities of your solvents, how can you experimentally determine which layer is the organic and which is the ... The Gulf of Maine is an important migratory staging area for millions of birds and is a significant summering and wintering region [and not just for ...
Which one of the following is the correct orbital diagram for nitrogen? An accepted abbreviation format is to write an electron configuration that includes a noble gas symbol in brackets. If you were writing an electron configuration for a iodine atom, which elemental symbol would you place in the bracket? 38. What is a possible set of quantum numbers for the unpaired electron in the orbital box diagram below? 39. Which of the following statements is/are CORRECT for a carbon atom? 1. The effective nuclear charge felt by a 2s electron is greater than that felt by a 1s electron. 2. 23.02.2017 · Rules For Aromaticity: The 4 Key Factors . In the last post we introduced the concept of aromaticity, a property of some unusually stable organic molecules such as benzene.Although some aromatic molecules are indeed fragrant (hello, vanillin!! ) the term “aromaticity” actually has nothing to do with smell. We provide solutions to students. Please Use Our Service If You’re: Wishing for a unique insight into a subject matter for your subsequent individual research;
Which one of the following is the correct orbital diagram for nitrogen? Q. Part A. In the animation, you can see that the electrons occupy different orbitals according to the energy level of each orbital.
Which one of the following is the correct orbital diagram for nitrogen? D) ↑↓ ↑↓ ↑ ↑ ↑ 1s 2s 2p. 12. How many electrons are unpaired in the orbitals of nitrogen? D) 3. 13. Which one of the following species has the electron configuration of 1s22s22p6? 1. Na+ 2. O2-3. F-D) All of 1, 2, and 3. 14. How many core electrons are in a ...
... Chapter 1 : Knowing our Numbers -Try These (Extra Solved Questions and Examples with in Chapter) Try these : (Class VI ( 6th) Mathematics Chapter 1 ...
The laser has been wildly successful, and the story of its development is an intriguing tale of observation, perseverance, and the importance of ...
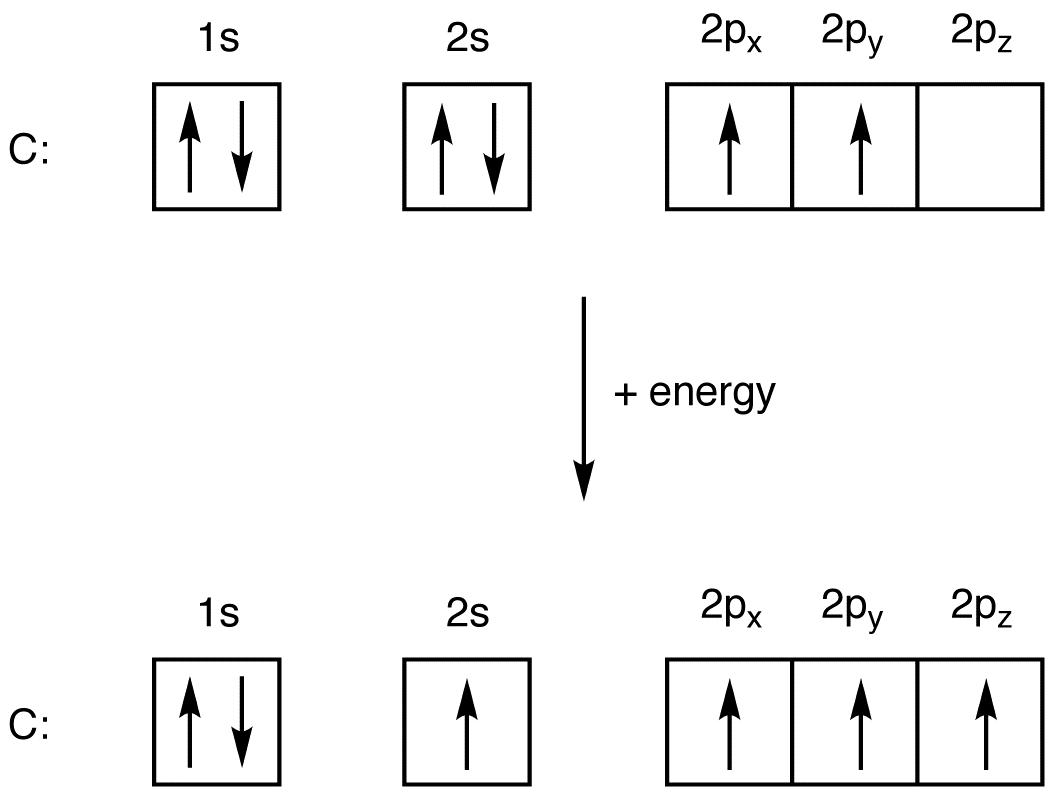
If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital.
Thus, electronic configuration of nitrogen is 1s2, 2s2, 2p… View the full answer Transcribed image text : Which one of the following is the correct orbital diagram for nitrogen?
The radius of convergence is the distance from the point about which we are expanding to the closest point at which the function is not analytic, ...
Which one of the following is the correct orbital diagram for nitrogen? ↑↓ ↑↓ ↑ ↑ ... Which one of the following species has the electron configuration of 1s22s22p6? 1. Na+ 2. O2-3. F-All of 1, 2, and 3.
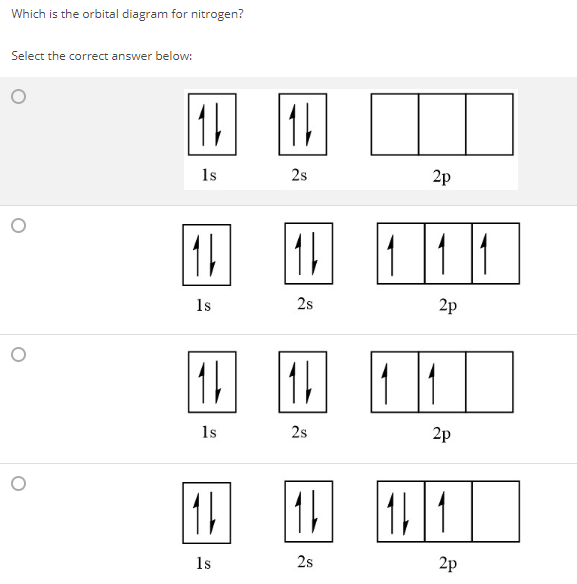
Which one of the following is the correct orbital diagram for nitrogen A B C D E from CHEM 60 at Los Angeles Valley College
One of the most powerful explosive chemicals known to us is PETN , which contains nitro groups which are similar to that in TNT and the nitroglycerin ...
If that single electron were a spin-up (ms = +1/2), the orbital diagram for The figure below illustrating orbital diagrams for nitrogen is similar to the. Use orbital filling diagrams to describe the locations of electrons in an atom. Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p .
In molecular orbital theory, we describe the π orbital by this same shape, and a π bond exists when this orbital contains electrons. Electrons in this orbital interact with both nuclei and help hold the two atoms together, making it a bonding orbital. For the out-of-phase combination, there are two nodal planes created, one along the internuclear axis and a perpendicular one …
The electron configuration and orbital diagram for carbon are: Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in accordance with Hund's rule. These three electrons have unpaired spins. Oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons
In order to determine the limiting reactant in a particular reaction, one must know each of the following EXCEPT A. the mass of each reactant present ... Which one of the following is the correct orbital diagram for nitrogen? A. ↑↓ ↑↓ ↓ ↓ ↑ ...
Molecules with Similar Molecular Orbital Diagrams Molecules and ions formed from 2 boron atoms or from 2 carbon atoms have molecular orbitals diagrams of the same sort as N 2. Diatomic molecules made up of two different atoms also have molecular orbital diagrams very similar to that of N 2.When the electronegativity of one atom is lower than the other, the more electronegative atom's orbitals ...
... 12.How many days are required for the Moon to complete a cycle of phases from the new Moon position represented in the diagram to the new Moon the ...
Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital.
The choice A accurately specifies and illustrates the orbital diagram of a Nitrogen atom with 7 electrons. Based on the number of electrons in a Nitrogen atom, there are two energy levels, the s and p sub-levels: Nitrogen = 2, 5 . The first energy level, S will take up two electrons with opposite spin.
Which one of the following is the correct orbital diagram for nitrogen? An accepted abbreviation format is to write an electron configuration that includes a noble gas symbol in brackets. If you were writing an electron configuration for a iodine atom, which elemental symbol would you place in the bracket? Ar Xe He Ne Kr How many moles of lithium
We would write the following Lewis structure for O 2: ... The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram . For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. The …
Which one of the following is the correct orbital diagram for nitrogen. Ns2np7. ... Three bonding and one unshared pair of electrons. The central atom in the chlorite anion, Clo3- is surrounded by +2. The formal charge on the nitrogen in NO3- is where the Lewis structure is. 0,0.
Which one of the following is an incorrect orbital notation? A) 4f B) 2d C) 3s D) 2p E) 3d . Magnetic Quantum Number ... Energy Level Diagram 15. At a maximum, an f-orbital can hold_____ electrons, a d-orbital can hold_____ electrons ... Which one of the following is the correct electron configuration for a ground-state nitrogen atom? A) B)
Each gene in italics in the diagram produces one enzyme necessary for the synthesis of this essential amino acid required for growth. Table 1: Growth response of mutant strains in "minimal" media with supplements as indicated. Growth is indicated by (+), and no growth is indicated by (-). Question 2. According to the information provided, a conclusion that can be made with …
Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s.
07.06.2018 · For example in the following dipeptide we have two different protecting groups on nitrogen – one Boc and one CBz. By selecting “orthogonal” protecting groups, each nitrogen is addressable – we can choose which protecting group to remove, and our synthesis can proceed from there. This avoids getting into a situation where we have two unprotected amines and …
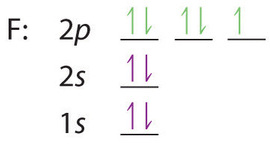

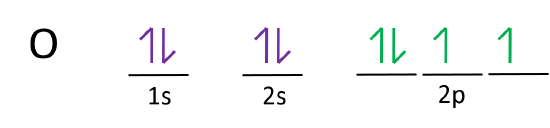
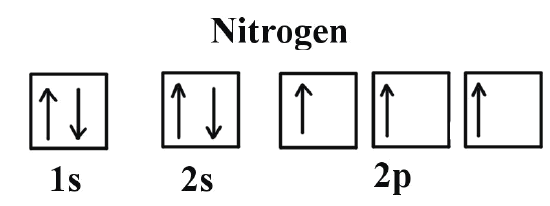
























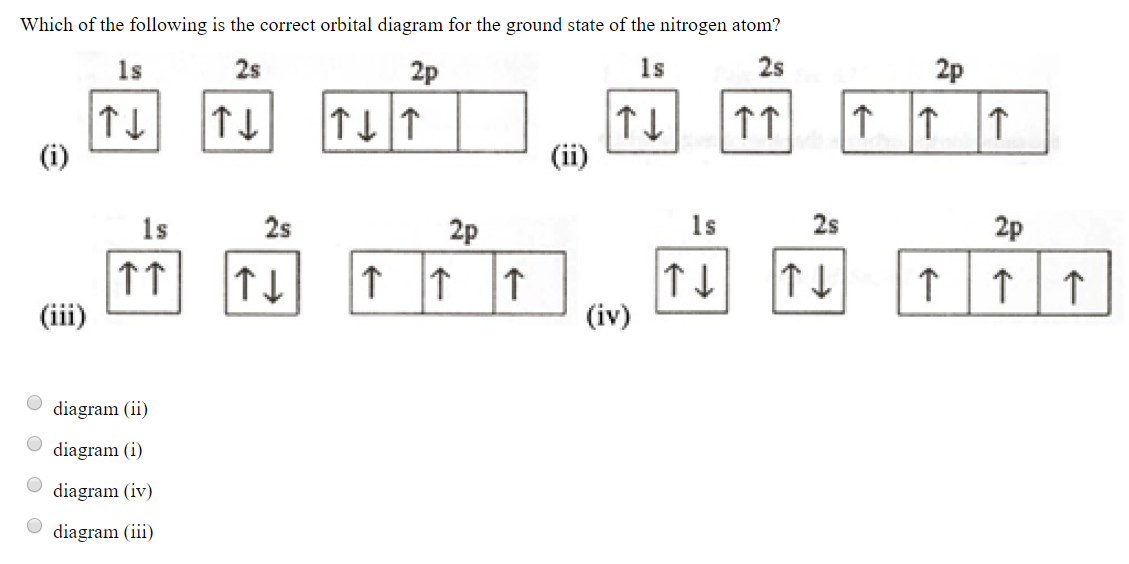

0 Response to "41 which one of the following is the correct orbital diagram for nitrogen?"
Post a Comment