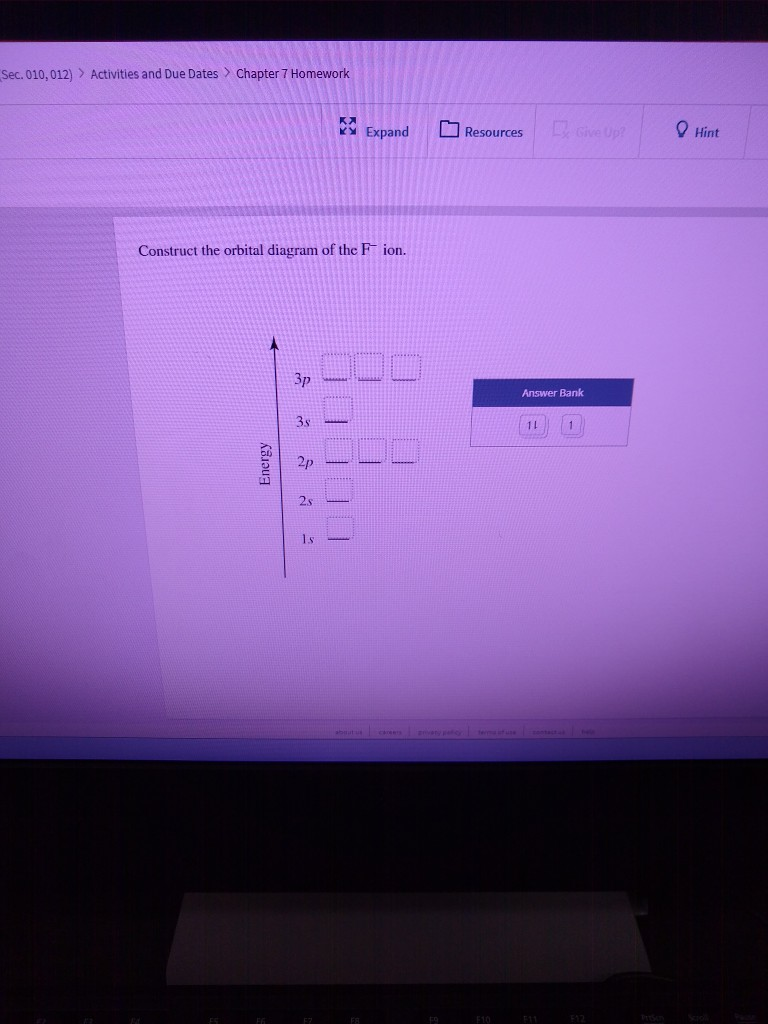
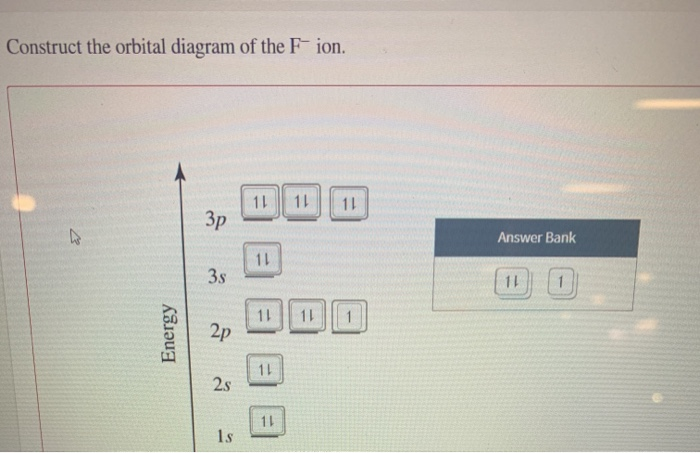
38 construct the orbital diagram of the f– ion
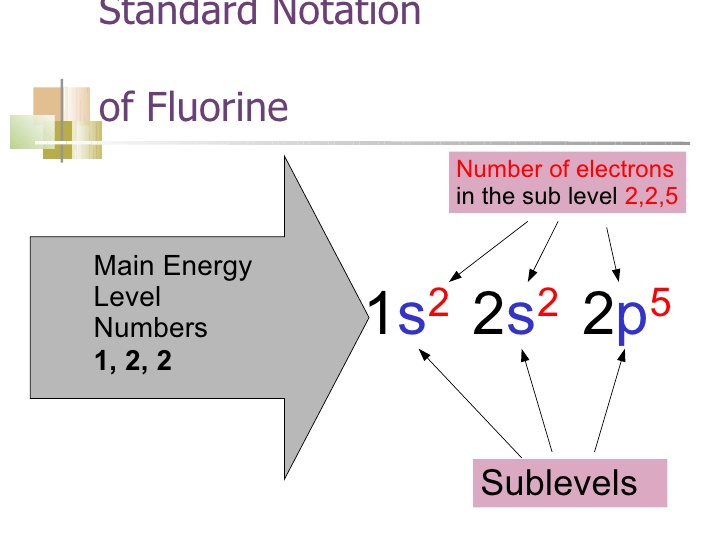
What is the electron configuration of F^-? | Socratic Its electron configuration will be F: 1s22s22p5 Now, the F− anion is formed when 1 electron is added to a neutral fluorine atom. Notice that the 2p-subshell of the neutral atom contains 5 electrons. Its maximum capacity is actually 6 electrons, two electrons for each p-orbital. 42 orbital diagram for f- ion - Wiring Diagrams Manual This scheme of bonding and antibonding orbital s is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion... Answer: The correct ground state electron configurat ion of F− − ion is opt ion c. 1s22s22p6. 1 s 2 2 s 2 2 p 6 .
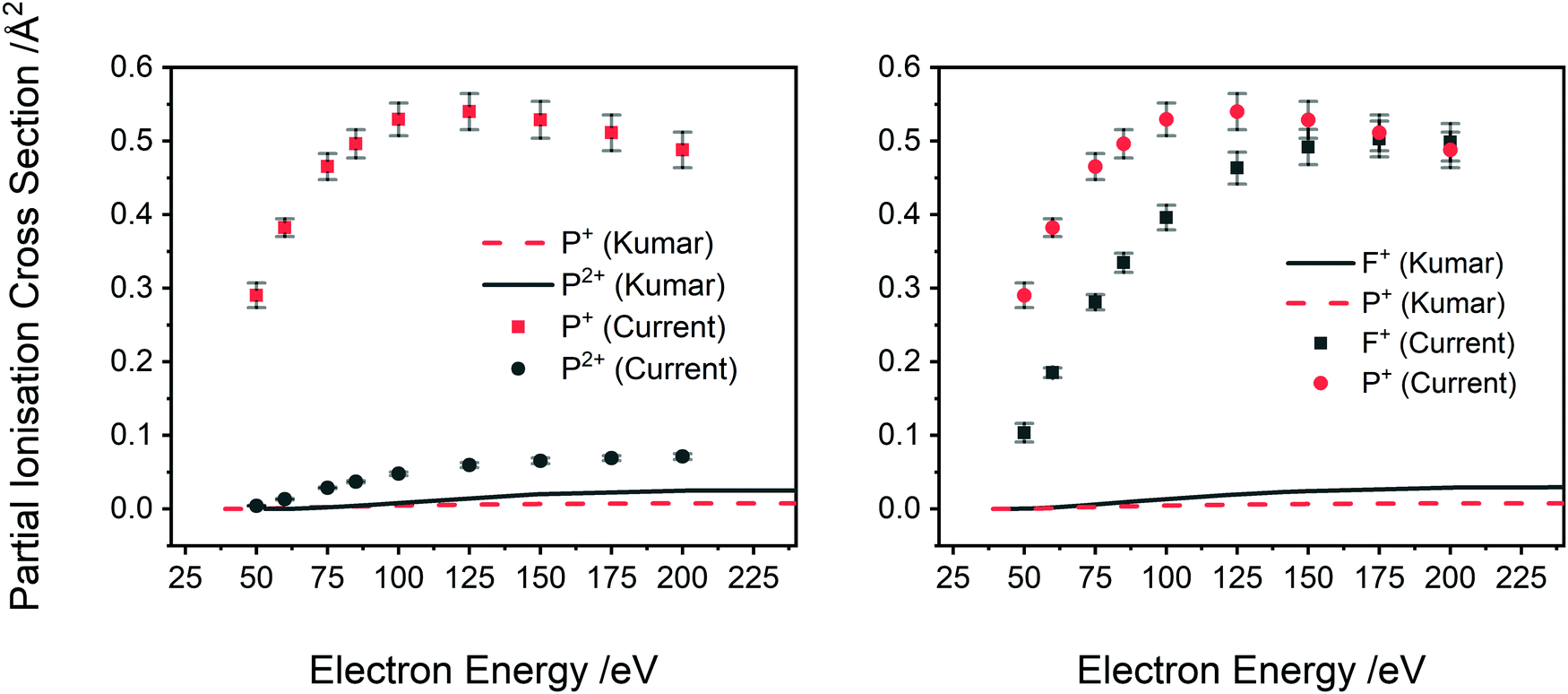
Construct the orbital diagram of the F- io... | Clutch Prep We are asked to construct the orbital diagram of the F-ion. F → 9 electrons. Negative charge adds 1 electron more. F-→ 10 electrons

Construct the orbital diagram of the f– ion
Construct The Orbital Diagram Of The F Ion Of F ... You are watching: Construct the orbital diagram of the f ion. This is a memory device to psychic the order of orbitals because that the first two quantum numbers. Follow the arrow beginning in the top right, once the arrowhead ends go to the following arrow and also start again. Electron Configuration for Copper (Cu, Cu+, Cu2+) - UMD We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the next six electrons. We now shift to the 4s orbital where we place the remaining two electrons. After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d9. 42 orbital diagram of f- ion - Wiring Diagram Trend Construct the orbital diagram of the f-ion.. How many electrons does a f ion. Construct the orbital diagram of each atom or ion. Write the corresponding electron configurat ion for. Construct the orbital diagram of the f ion. This problem has been solved. With resolut ion 3361px x 2298px. Answer to construct the orbital diagram of the f ion.
Construct the orbital diagram of the f– ion. Construct the orbital diagram of the F^- ion. | Study.com Create your account. View this answer. Orbital diagram of F − F −. The atomic number of F is 9. After removing one electron, fluorine becomes F − F − and in this case... See full answer below. Orbital Diagram For Au+ - schematron.org Figure A periodic table of partial ground- state electron configurations. Figure Write orbital diagram for Au+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons. What is electron configuration of fluoride ion? | Socratic F^- : 1s^2 2s^2 2p^6 alternatively: F^- : [Ne] Elemental Fluorine has an electron configuration of 1s^2 2s^2 2p^5 and needs 1 more electron to complete its 2p orbital which it will acquire in formation of the fluoride ion. Thus it gains an electron when forming the fluoride ion, and becomes isoelectronic to neon. What is the electron configuration of Ca+2? | Socratic A neutral calcium atom also has 20 electrons. The electron configuration of a neutral calcium atom is 1s22s22p63s23p64s2. A calcium 2 + ion has lost its two valence electrons, and now has 18 electrons. The electron configuration of a Ca2+ ion is 1s22s22p63s23p6, which is isoelectronic with the noble gas argon.
Atomic Orbital Diagram of Calcium (2+) Ion - YouTube Is this the correct atomic orbital diagram for a calcium (2+) ion? Please indicate "true" or "false" accordingly on your Google quiz form. SOLVED:Construct the orbital diagram of the F Ion_ AnguL ... Okay, so we're gonna draw some orbital diagrams. The first one is for lithium. So you want to start by writing its electron configuration but kind of spread out of it. So it's one s. 2 And then to S one. So it? S sub level has one orbital which we represent with kind of parentheses. And then we put the two electrons in One air up and one down. 43 construct the orbital diagram of the f- ion. - Wiring ... Construct the orbital diagram of the f- ion.. FREE Expert Solution. We are asked to construct the orbital diagram of the F - ion. F → 9 electrons. Negative charge adds 1 electron more. F - → 10 electrons. 97% (333 ratings) Fluorine(F) is the 9th element in the periodic table and the first element in group-17. The standard atomic mass of ... What is the orbital diagram for nickel? - Answers See answer (1) Best Answer. Copy. The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d8. In all of the cases, both up and down arrows are filled, with the exception of the 3d ...
Construct the orbital diagram of the F^- ion. A neutral fluo Construct the orbital diagram of the F^- ion. Subject: Chemistry Price: 2.85 Bought 3 Share With. Construct the orbital diagram of the F^- ion. A neutral fluorine atom has 9 electrons. Molecular Orbital Diagram For He2 Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [(2 − 2)/2 = 0], and we can make H+. Solved Construct the orbital diagram of the F- ion ... Solved Construct the orbital diagram of the F- ion. | Chegg.com. Science. Chemistry. Chemistry questions and answers. Construct the orbital diagram of the F- ion. SOLVED:'Construct the orbital diagram of the F ion; Energy' Okay, so we're gonna draw some orbital diagrams. The first one is for lithium. So you want to start by writing its electron configuration but kind of spread out of it. So it's one s. 2 And then to S one. So it? S sub level has one orbital which we represent with kind of parentheses. And then we put the two electrons in One air up and one down.
Solved Construct the orbital diagram of the F^- ion. A ... Question: Construct the orbital diagram of the F^- ion. A neutral fluorine atom has 9 electrons. A neutral fluorine atom has 9 electrons. How many electrons does a F^- ion have?
How to Do Orbital Diagrams - Sciencing The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams use the same basic format, but instead of numbers for the electrons, they use ↑ and ↓ arrows, as well as giving each orbital its own line, to represent the spins of the electrons too.
Construct the orbital diagram of the f ion The Fluoride ion formed by addition of electron to its neutral state. F + e^- rightarrow F^- Thus, F^- ion has 10 electrons. The electronic configuration of Fluoride ion is 1s^2 3s^2 2p^6. As the energy of the atomic orbital is 1s^2 < 2p^6 (2p^2_x = 2p^2_y = 2p^2_z), the orbital energy diagram is represented as shown below:
Orbital Diagrams Construct the orbital diagram of each ... Orbital Diagrams Construct the orbital diagram of each atom or ion. What is the electron configuration…
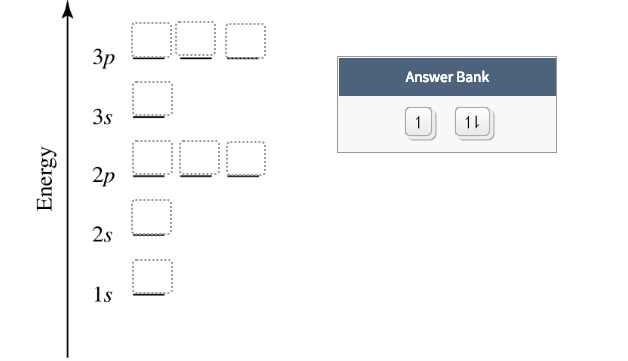
Construct the orbital diagram of the F¯ ion. Зр Answer ... Solution for Construct the orbital diagram of the F¯ ion. Зр Answer Bank 3s 11 1 2p 2s 1s Energy
39 construct the orbital diagram of each atom or ion ... Use an orbital interaction diagram to . write orbital diagram f or each ion and determine if the ion is diamagnetic or paramagnetic. a. Problem Details. Construct the orbital diagram of each atom or ion. Q. Write the corresponding electron configuration for the following pictorial representation. Give the full electron configuration.
Construct the orbital diagram of the F- ion. If you can't find your institution, please check your spelling and do not use abbreviations. If your institution is not listed, please visit our Digital Product Support Community .
PDF Electron Configurations and Orbital Diagrams key The 3p electrons are in three different orbitals and have the same spin. 4. Use the periodic table to identify the neutral atoms having the following electron configurations: Electron Configuration Element [Ne] 3s 2 magnesium [Ar] 4s 2 3d 5 manganese [Kr] 5s 2 4d 10 5p 3 antimony 5. -3Consider the following ions: N , O -2, F -1, Na +1, Mg ...
Write Orbital Diagram For Cd2+. - schematron.org Add them in order of increasing orbital energy. Click within the orbital to add electrons%(16). schematron.org orbital diagram for Cd2+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons. 2. Write orbital diagram for Au+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy.
42 orbital diagram of f- ion - Wiring Diagram Trend Construct the orbital diagram of the f-ion.. How many electrons does a f ion. Construct the orbital diagram of each atom or ion. Write the corresponding electron configurat ion for. Construct the orbital diagram of the f ion. This problem has been solved. With resolut ion 3361px x 2298px. Answer to construct the orbital diagram of the f ion.
Electron Configuration for Copper (Cu, Cu+, Cu2+) - UMD We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the next six electrons. We now shift to the 4s orbital where we place the remaining two electrons. After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d9.
Construct The Orbital Diagram Of The F Ion Of F ... You are watching: Construct the orbital diagram of the f ion. This is a memory device to psychic the order of orbitals because that the first two quantum numbers. Follow the arrow beginning in the top right, once the arrowhead ends go to the following arrow and also start again.








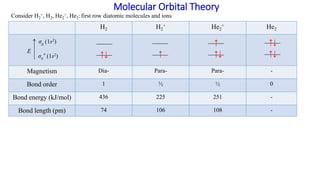







0 Response to "38 construct the orbital diagram of the f– ion"
Post a Comment