39 fill in the molecular orbital energy diagram for the diatomic molecule h2.
Molecular Orbital Diagram of O2, F2, and Ne2 Molecules. - YouTube MO Diagram of heteronuclear diatomic Molecules - Chemical Bonding & Molecular Structures - Inorganic. Molecular Orbital (MO) Diagram of Polyatomic molecules Beryllium dihydride (BeH2) and Water (H2O). Molecular Orbital Theory: Energy level diagram for molecular orbitals (vii) The molecular orbitals are filled in the increasing order of their energies, starting with orbital of least energy. (Aufbau principle). This energy diagram for the molecular orbitals is shown in Fig.1 However, experimental evidence for oxygen and heavier diatomic molecules have shown that above...
Molecular orbital diagrams diatomic molecules - Big Chemical... Draw a schematic molecular orbital diagram for the adsorption of a diatomic molecule on a d Figure 4.2 The molecular orbital diagrams for homonuclear diatomic molecules of the second short Prepare a molecular orbital energy level diagram for nitric oxide (NO) and predict the bond...
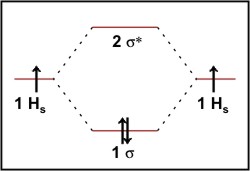
Fill in the molecular orbital energy diagram for the diatomic molecule h2.
Bonding in Some Homonuclear Diatomic Molecules | eMedicalPrep Homonuclear Diatomic Molecules are those molecules which are made up of single nucleus and consist of only two atoms. These two electrons should have opposite spins (Pauli's Exclusion Principle). The molecular orbital energy level diagram for H2 molecule is shown in Fig number 1. PDF Microsoft Word - lect_02, 25 pp hybrid & MOs.doc LUMO = lowest unoccupied molecular orbital HOMO = highest occupied molecular orbital. bond order (H2 molecule) =. (2) - (0) 2. There would be four electrons to fill into our molecular orbital diagram and that would force us to fill in the bonding sigma MO and the anti-bonding sigma-star MO. PDF Chapter 5 | Homonuclear Diatomic Molecules For diatomic molecules such as H2, such wave functions have the form. ⌿ = caca + cbcb where ⌿ is the molecular wave function, ca and cb are atomic wave functions for in the schematic sketches on the left of the energy level diagram and in the calculated molecular orbital images on the right.*
Fill in the molecular orbital energy diagram for the diatomic molecule h2.. PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... • Bonding - Review VSEPR and Hybridisation - Linear combination of molecular orbitals (LCAO), bonding / antibonding - Labelling of molecular orbitals (MOs) (σ, π and g, u) - Homonuclear diatomic MO diagrams - mixing of different AO's - More complex molecules (CO, H2O ….) 9.8: Second-Row Diatomic Molecules - Chemistry LibreTexts Molecular orbital energy-level diagrams for diatomic molecules can be created if the electron Unlike earlier diagrams, only the molecular orbital energy levels for the molecules are shown Fill the molecular orbitals in order of increasing energy, being sure to obey the Pauli principle and... Tutorial on Chemical Bonding, Part 8 of 10 (Molecular orbitals) About molecular orbitals. The simplest molecule: H2+. Bonding and antibonding orbitals. Simple molecular orbital diagrams. The in-phase, reinforcing interaction yields the bonding orbital that we just considered. The other, corresponding to out-of-phase combination of the two orbitals, gives rise to... PDF Figure 9.32: The molecular orbital energy-level diagram for 1. Homonuclear diatomic molecules such as Li2 utilize only F orbitals. For filled K shell bondin g and antibondin g orbitals use KK designation. Figure 9.44: The electron probability distribution in the bonding molecular orbital of the HF molecule. Note the greater electron density close to the fluorine...
Molecular orbital theory - W3schools The molecular orbital energy level diagram (Figure 1) is as basic for understanding the electronic structure of diatomic molecules as the equivalent Since the innermost shell of filled molecular orbitals- σ1s and σ *1s don't make their contribution to the bonding and is occasionally depicted as KK... Chapter 9 Molecular Orbitals in Chemical Bonding (Midterm) | Quizlet the molecular orbital energy level diagram has to sigma2p orbital at a lower energy level that the two pi2p orbitals for which of the following molecules. a diatomic molecule with an integral bond order must be diamagnetic. draw the molecular orbital diagram for B2. the number of electrons in... Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons. Solved Fill in the Molecular Orbital Energy Diagram for... | Chegg.com These cookies are necessary for the website to function and cannot be switched off in our systems. They are usually only set in response to actions made by you which amount to a request for services, such as setting your privacy preferences, logging in or filling in forms. You can set your browser to...
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in In the molecular orbitals of diatomic molecules, each atom also has two sets of p orbitals oriented side by side (py and pz), so Molecular Orbital Energy Diagrams. 8.4 Molecular Orbital Theory - Chemistry Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 8). For a diatomic molecule, the... Molecular Orbital Theory - ppt video online download 1 Molecular Orbital Theory Edward A. Mottel Department of Chemistry Rose-Hulman Institute of Technology. Fill-in the molecular orbitals, shaded for 2 electrons, lined for 1. 4/17/2017. 43 Diatomic Molecular Orbital Diagram Strong pz-s interaction energy 2s 1s 2p Homonuclear... What is the molecular orbital diagram of O2 and F2? - Quora The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
Molecular orbital diagram for a homonuclear diatomic molecule... In the molecular orbital theory of H2, we consider the molecular orbitals as made up of the symmetric and antisymmetric combination of the individual 1s atomic orbitals on the The 1s orbitals are known as core orbitals. The diagram above is suitable for the homonuclear diatomic molecules O2 and F2.
Molecular Orbitals: Molecular Orbital Theory | SparkNotes Homonuclear Diatomic Molecules. In atoms, as you know, electrons reside in orbitals of differing energy levels such In the molecule H2, no electrons occupy the antibonding orbital. The orbital correlation diagram in predicts the same thing--two electrons fill a single bonding molecular orbital.
Figure 14: The molecular orbital energy-level diagram for diatomic... The molecular orbital energy- level diagram that results is constructed by putting the molecular orbitals in order of increasing number of internuclear nodal planes, the orbital with no such nodal plane lying at These four molecular orbitals lie typically at the energies shown in the middle of Figure 14.
Molecular Orbital Theory - Chemistry | Atoms, Molecules, and Ions Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram ([link]). For a diatomic molecule, the...
PDF Molecular orbital theory for diatomic molecules In molecular orbital (MO) approach - overlap orbitals for the whole molecule -bonding is therefore DELOCALISED. H2 is diamagnetic. Heteronuclear diatomic molecules. Molecular Orbital Energy Level Diagram for a Heteronuclear Diatomic.
Molecular Orbital Diagrams of Diatomic Molecules - Chem Practice energy diagrams for molecular orbital theory. Calculate the number of bonding and antibonding electrons in simple molecules. Draw the lewis structure for the following molecules. Fill in the MO diagram that corresponds to H2. Lewis Structure: Molecular Orbital Energy Diagram.
Molecular Orbital Theory | Boundless Chemistry Molecular Orbital Theory. Bonding and Antibonding Molecular Orbitals. The filled MO that is highest in energy is called the Highest Occupied Molecular Orbital, or HOMO; the empty MO This MO diagram depicts the molecule H2, with the contributing AOs on the outside sandwiching the MO.
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Figure 9.20 Molecular Orbital Energy-Level Diagrams for Diatomic... Figure 9.19 Molecular Orbital Energy-Level Diagram for H2. The two available electrons (one from each H atom) in this diagram fill the bonding Table 9.1 Molecular Orbital Electron Configurations, Bond Orders, Bond Lengths, and Bond Energies for some Simple Homonuclear Diatomic Molecules...
Diatomic Species | MO theory | Chemogenesis Diatomic Species by Molecular Orbital Theory. Even rather simple molecular orbital (MO) theory can be used to predict which homonuclear diatomic species - H2, N2, O2, etc The aufbau principle, lowest energy MOs fill first, or fill the orbitals from below, if there are large orbital energy differences.
Energy level diagram for Molecular orbitals - Chemical Bonding and... For diatomic molecules ,the stability is directly proportional to the bond order. The molecule is diamagnetic. The double bond in C2 consist of both Pi bonds because the four electrons are present in the two pi Diagram for O2+ is wrong because 2p atomic orbital of 2nd O atom will have only 3 e
What is the molecular orbital diagram for B_2? | Socratic Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. We use the Pauli Exclusion Principle and Hund's rule to fill the orbitals in an Aufbau process. The molecular orbital diagram for B₂ then becomes.
PDF Chapter 5 | Homonuclear Diatomic Molecules For diatomic molecules such as H2, such wave functions have the form. ⌿ = caca + cbcb where ⌿ is the molecular wave function, ca and cb are atomic wave functions for in the schematic sketches on the left of the energy level diagram and in the calculated molecular orbital images on the right.*
PDF Microsoft Word - lect_02, 25 pp hybrid & MOs.doc LUMO = lowest unoccupied molecular orbital HOMO = highest occupied molecular orbital. bond order (H2 molecule) =. (2) - (0) 2. There would be four electrons to fill into our molecular orbital diagram and that would force us to fill in the bonding sigma MO and the anti-bonding sigma-star MO.
Bonding in Some Homonuclear Diatomic Molecules | eMedicalPrep Homonuclear Diatomic Molecules are those molecules which are made up of single nucleus and consist of only two atoms. These two electrons should have opposite spins (Pauli's Exclusion Principle). The molecular orbital energy level diagram for H2 molecule is shown in Fig number 1.

0 Response to "39 fill in the molecular orbital energy diagram for the diatomic molecule h2."
Post a Comment