42 xef molecular orbital diagram
molecule is XeF 4 Hybridization of Xe atom is sp 3 d 2 ... 21 If we look at H 2, we see that each hydrogen atom has a 1s atomic orbital that is half-filled. Remembering that orbitals are mathematical functions, they can combine by adding or subtracting to give two new combinations which we call molecular orbitals. On the other hand, the energy of the H 2 molecule with two electrons in the antibonding orbital is higher than two separate H-atoms. pem.pm › d155c9-xef4-molecular-orbital-diagramxef4 molecular orbital diagram - pem.pm An MO diagram is a descriptive instrument that is particularly used to explain the formation of chemical bonds in molecules with the help of molecular orbital theory. Dear Student, a) b)In,XeF4,the central atom,Xe,has eight electrons in its outermost shell. Determine whether each is paramagnetic or diamagnetic.
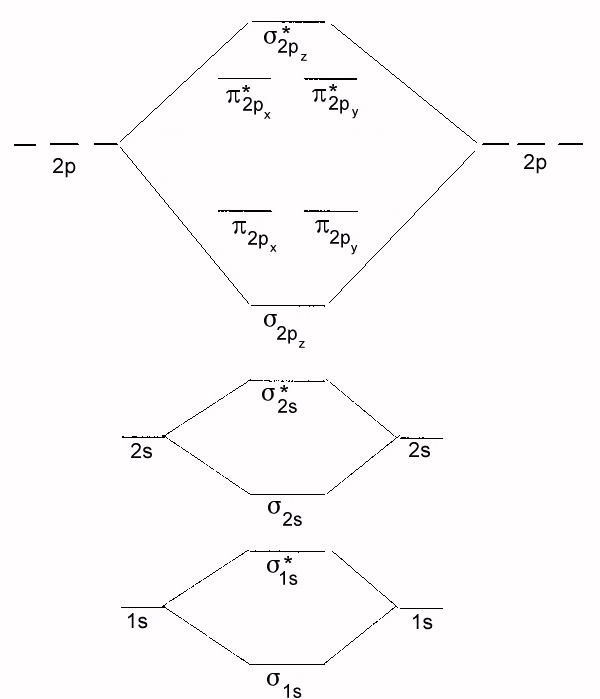
PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
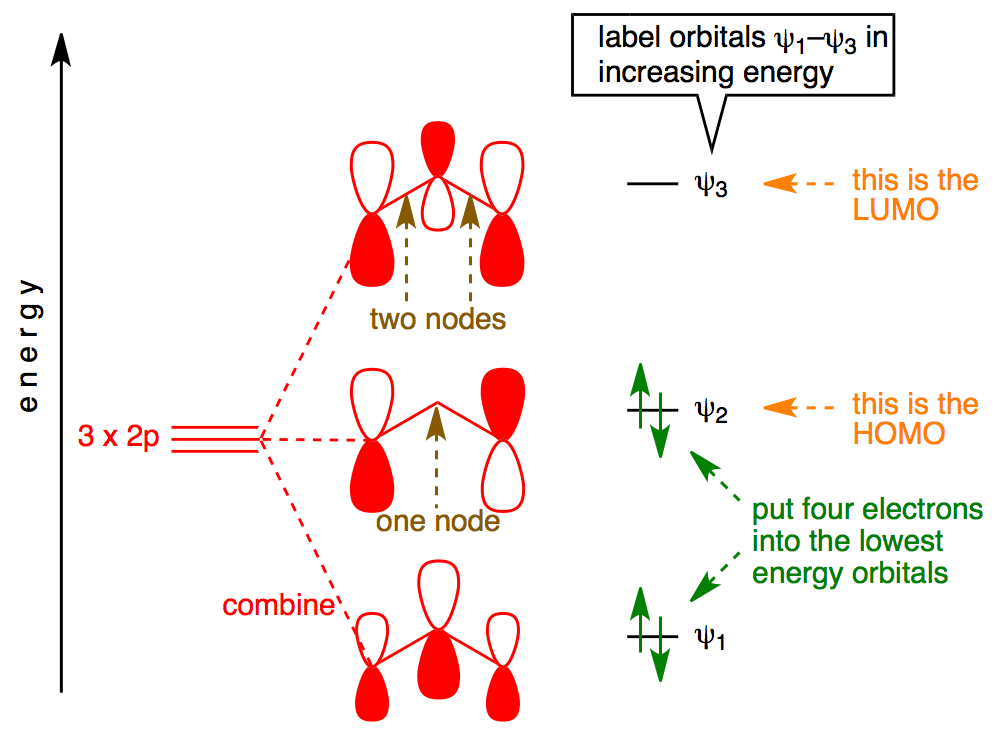
Xef molecular orbital diagram
Photoionization from the Xe 4d orbitals of XeF 2 (Journal ... The Xe 3d-ϵf continuum shape resonances dominate the photoionization cross sections of both the atom and molecule, but prominent resonances appear in the XeF{sub 2} cross section due to nominal excitation of Xe 3d and F 1s electrons to the lowest unoccupied molecular orbital (LUMO), a delocalized anti-bonding MO. Xef2 Lewis Structure, Polarity, Hybridization and shape Xef2 Lewis Structure, Polarity, Hybridization and shape. XeF2 is an abbreviation for the chemical compound Xenon Difluoride. It is a powerful fluorinating as well as an oxidizing agent. Apart from XeF2, there are other Xenon compounds such as XeF4 ( Xenon Tetrafluoride) and XeF6 ( Xenon Hexafluoride). Out of these compounds, XeF2 is the most ... Hybridization of XeF2 - Hybridization of Xe in Xenon ... The central atom Xe has 2 bond pair and 3 lone pairs around it. During hybridization, Xenon will form two sigma bonds with two fluorine atoms. There are three hybrid orbitals that contain the lone pairs and they do not form any bonds. XeF2 Molecular Geometry And Bond Angles XeF2 molecular geometry is linear.
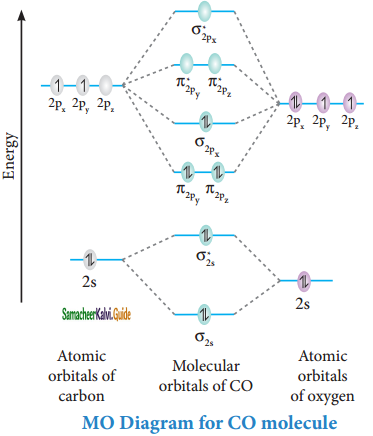
Xef molecular orbital diagram. Molecular Orbital Diagram Maker ©2022 Prof Adam J Bridgeman | close window : ©2022 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window Formation of twelve-fold iodine coordination at high ... Jan 20, 2022 · The high pressure and the presence of N 6 rings reduce the energy level of the 5d orbital of iodine, making them part of the valence orbital. … inorganic chemistry - Chemistry Stack Exchange How to get those correlations, is quite a fair question. Well, I got it from this diagram in Orbital Interactions in Chemistry, 2nd ed. by Albright et al.The correlation diagram 14.4 should be self-explanatory, hopefully (you can ignore the pictures of the MOs, 14.5 and 14.6).. But if you want to obtain it without flipping through every book in the library, this is how you need to do it. PDF Chem 130 - Second Exam Key - DePauw University the molecular orbitals. The complete molecular orbital diagram shows that (a) sulfur is on the left and oxygen is on the right, that (b and c) sulfur's valence shell is 3s23p4 and oxygen's valence shell is 2s22p4, and that (d) the 12 total valence electrons fill the molecular orbitals from the bottom-to-top, filling each
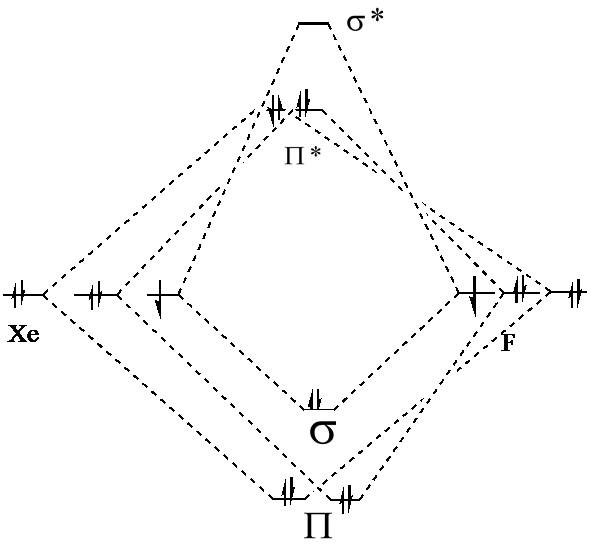
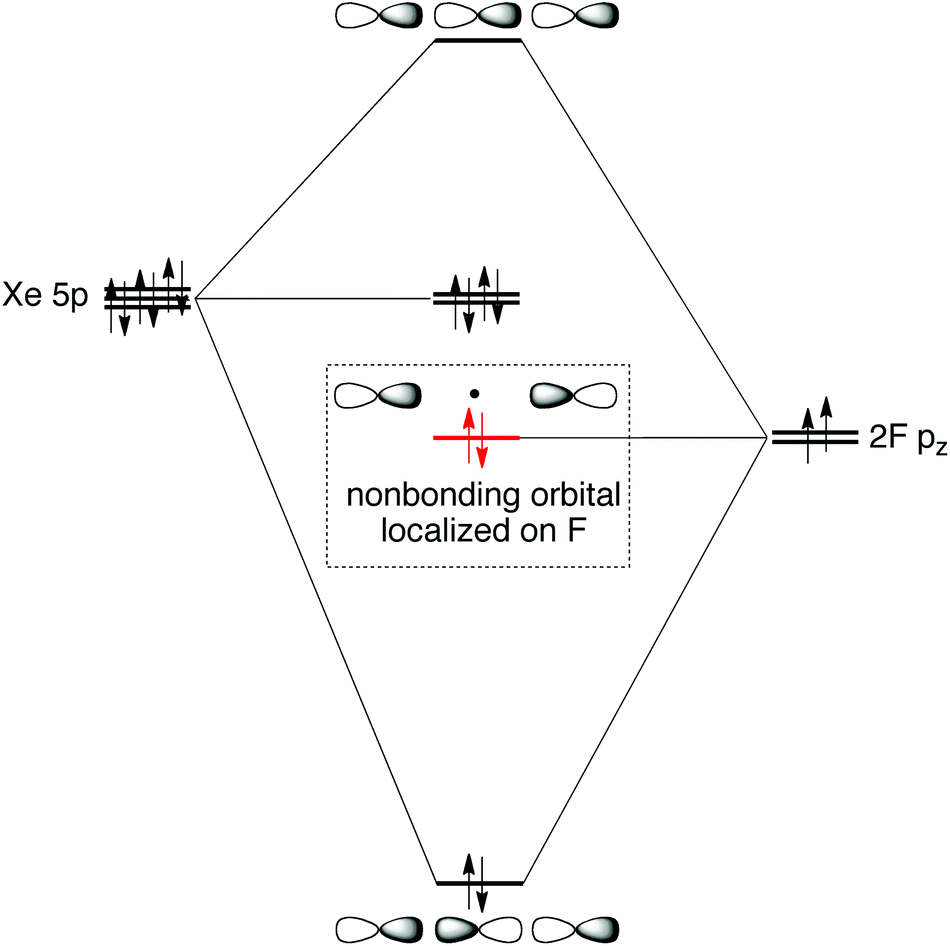
› watchMOT Molecular Orbital Diagram Of XeF2 - YouTube #MOT Molecular Orbital Diagram Of #XeF2 #Xenon Di FluoRide In English#NobleGases#InertGases#Chemistry#XenonDiFluoride#Bonding#MolecularOrbitalDiagram#Jee #Neet people.physics.anu.edu.au › ~mxk121 › researchXeF2 Molecular Orbital - Australian National University F − Xe+− F−↔ F − Xe+− F− which means that the Xe−F bonds have a half-ionic and half-covalent character. The electronic configuration of XeF2in the ground electronic state is given in the following: (8σg)2(5σu)2(9σg)2(6σu)2(4πu)4(3πg)4(10σg)2(5πu)4(7σu)0. The lowest vacant orbital, 7σu, which has an antibonding character consists Solved Although KrF+ and XeF+ have been studied, KrBr+ has ... Although KrF + and XeF + have been studied, KrBr + has not yet been prepared. For KrBr +:. a)Propose a molecular orbital diagram showing the interactions of the valence shell s and p orbitals to form molecular oribtals XeF4 Molecular Geometry - Science Education and Tutorials Xenon atom has s, p, and d orbitals. The fluorine atom has s and p orbital. The sp3d2 hybridization of the XeF4 molecule is formed when one S orbital, three p orbital, and two d orbitals join together to form a molecular orbital. Molecular Geometry Notation for XeF4 Molecule : Determine the form of XeF4 molecular geometry using VSEPR theory.
XeF2 bonding - YouTube In this screencast, Andrew Burrows uses molecular orbital theory to explain the bonding in xenon difluoride. ... molecule is XeF 4 Hybridization of Xe atom is sp 3 d 2 ... When atomic orbitals are combined to give molecular orbitals, the number of molecular orbitals formed equals the number of atomic orbitals used. A molecular orbital (like an atomic orbital) can contain no more than two electrons (Pauli Exclusion Principle), and are filled starting with the lowest energy orbital first. AP Chemistry- Practice Bonding Questions for Exam - Quia The following molecular orbital diagram may be used for the following problems. For oxygen and fluorine, the σ 2p orbital should be lower in energy than the π 2p. However, the diagram will still yield correct bond order and magnetic behavior for these molecules. halides - What is the molecular structure of xenon ... Experimental evidence. The structure of xenon hexafluoride ($\ce{XeF6}$) has always been controversial; it is a famously strong oxidising agent, so experimental studies of it have been hampered by difficulties in the isolation and storage of pure samples.Early studies indicated that the structure of $\ce{XeF6}$ showed deviations from octahedral symmetry.
Inorganic Chemistry 411/511 Construct an MO diagram for N2. Show atomic and molecular orbital levels with appropriate energies and symmetry labels, and indicate the electron filling.6 pages
PDF Molecular Structure of XeF II. Internal Motion and Mean ... Molecular Structure of XeF e. II. Internal Motion and Mean Geometry Deduced by Electron Diffraction * L. S. BARTELL AND R. M. GAVIN, JR. t Department of Chemistry, University of Michigan, Ann Arbor, Michigan (Received 8 September 1967) The distribution of internuclear distances ingaseous XeF. exhibits unusually diffuse XeF. bonded and F-F ...
Hybridization of XeF6 - Hybridization of the Xenon atom in ... XeF 6 has seven electron pairs. It consists of 6 bond pairs and one lone pair. Xenon has 8 electrons in its valance shell and it forms six bonds with the fluorine atoms. When the fluorides of xenon have formed the electrons in the valence shell of xenon get unpaired and are promoted to vacant 5d orbitals. XeF 6 Molecular Geometry And Bond Angles
PDF Hybridization and Molecular Orbital (MO) Theory molecular shapes based on valence electrons, lewis dot structures and electron repulsions. •Molecular orbital theory (MO) - a molecule is formed by the overlap of atomic orbitals to form molecular orbitals, electrons are then distributed into MOs. A molecule is a collection of nuclei with the orbitals delocalized over the entire molecule .
Xef4(Xenon Tetrafluoride) Molecular Geometry, Lewis ... But when this atom is in an excited state, two electrons in the p-orbitals move to d-orbitals; as a result, there are four unpaired electrons in total. Out of which, two are in p-orbitals, and the other two unpaired electrons are in d-orbitals. These hybridized orbitals lead to sp3d2 hybridization in XeF4. XeF4 Molecular Geometry
XeF2 Lewis Structure, Molecular Geometry, Hybridization ... XeF2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram XeF2 is a covalent inorganic halide formed by the inert gas xenon and the halogen fluorine. This is an active solvent and is found to be soluble in different fluorides like HF and bromine pentafluoride.
Answered: Sketch the molecular orbital energy… | bartleby The existence of compounds of the noble gases was once a great surprise and stimulated a great deal of theoretical work. Sketch the molecular orbital energy level diagram for XeF and deduce its ground-state electron configurations.
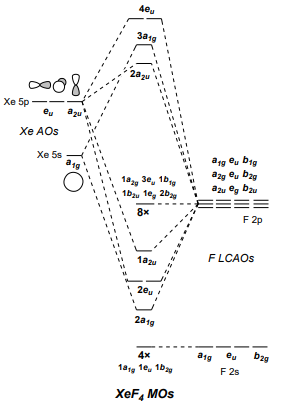
› homework-help › questions-andSolved Create a molecular orbital diagram for XeF4 | Chegg.com Create a molecular orbital diagram for XeF4 assuming the F atoms only participate in sigma bonding with the center. Indicate corresponding symmetry. (For Xe, E5s=-23.4 eV, E5p= -12.6 eV, E5d= -3 eV; for F E2s= -40.2 eV and E2p= -18.7 eV)
techiescientist.com › xef4-lewis-structureXeF4 Lewis Structure, Molecular Geometry ... - Techiescientist Mar 21, 2022 · MO Diagram of XeF4 An MO diagram is a descriptive instrument that is particularly used to explain the formation of chemical bonds in molecules with the help of molecular orbital theory. When atoms combine with other atoms to make molecules, some of the atomic orbitals adds up to form molecular orbitals which are the same in number.
Xenon tetrafluoride (XeF4) - D4h Symmetry - ChemTube3D Click the Symmetry Operations above to view them in 3D. XeF 4 belongs to the D 4h Point group and contains; One C 4 rotation axis, one C 2 rotation axis (equivalent to C 42 ), Four C 2 axes perpendicular to the C 4 axis. 4σ planes of symmetry,one σ h plane. One S 4 axis.
Lecture 4 d orbitals - Bonding in Molecules Bonding in Hypervalent Molecules. XeF ... d orbitals improve bonding, but we can explain the stability of XeF ... MO diagram for octahedral AH.31 pages
VSEPR Theory & Molecule Shapes - Video ... - Study.com Nov 21, 2021 · Determine the molecular shape of the molecule whose Lewis dot diagram is shown below. ... Molecular Orbital ... ClO_3^-, XeF_2, BrO_2^-. Determine the molecular geometries of the following ...
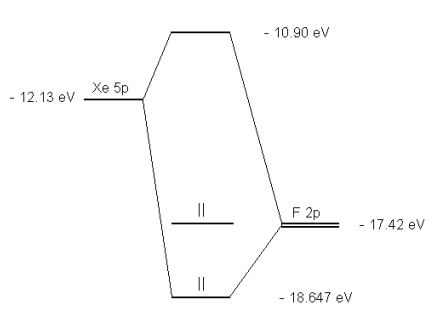
PDF Mathcad - XEF2MOT The molecular orbital diagram for this system is shown below. Is the molecule stable? The diagram shows two electrons each in the bonding and non-bonding orbitals for a total energy of -72.14 eV. The energy of the isolated atoms is 2(-12.13 eV) + 2(-17.42 eV) = - 59.1 eV.
Van der Waals heterostructures for spintronics and opto ... Jul 19, 2021 · The large variety of 2D materials and their co-integration in van der Waals heterostructures enable innovative device engineering. In addition, their atomically thin nature promotes the design of ...
tessilarreda.it Mar 20, 2022 · email protected] [email protected]
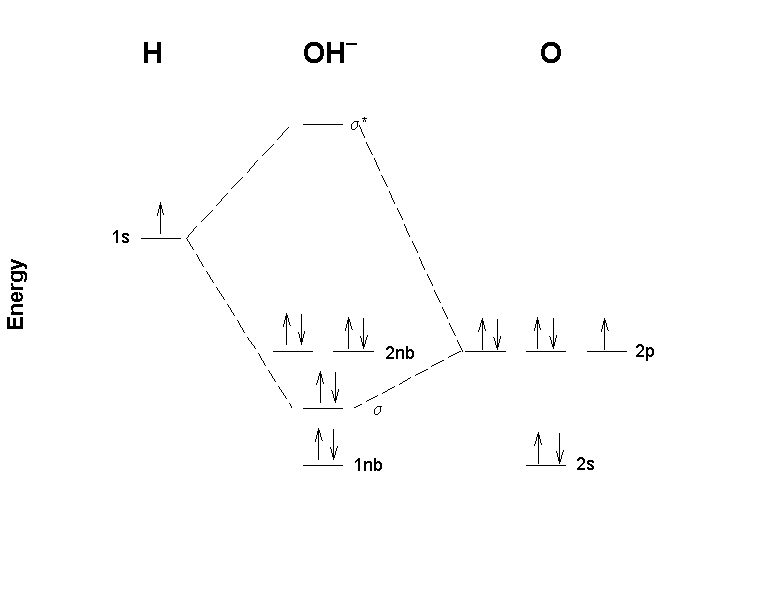
General Chemistry Questions a. it has such a low molecular weight. b. it is rather dense. c. the O-H single bond has a high bond energy. d. it has many relatively strong hydrogen bonds. e. it dissolves both ionic and covalent compounds. 8. The triple point is a. an end to the liquid-gas line in a phase diagram. b.
PDF Inorganic Chemistry with Doc M. - Creighton University Molecular orbital diagram for square planer XeF4 6. Using p-orbitals for σ-bonding: molecular orbital diagram for trigonal planer BF3 1. Symmetry adapted linear combinations (SALC) of bonding group atomic orbitals For molecules where the central atom is bonded to more than one other B group such as the
Square planar molecular geometry - Wikipedia A general d-orbital splitting diagram for square planar (D 4h) transition metal complexes can be derived from the general octahedral (O h) splitting diagram, in which the d z 2 and the d x 2 −y 2 orbitals are degenerate and higher in energy than the …
Molecular Structure Practice Problems Answers The molecular orbital diagram for ClO - is given below: The basis orbitals for Cl are 3s and 3p and for O are 2s and 2p. Z* for O 2s and 2p orbitals are similar so the AOs start at nearly the same energy. For the Cl 3s and 3p orbitals the two Z* values are quite different so the initial energies are more separated.
Hybridization of XeF4 - Explanation, Structure and ... These orbitals transfer to complete the empty 5d orbitals in the process of making the XeF 4. This results in 4 unpaired hybridized electrons which consist of 2 in 5p and 2 in 5d orbitals. So, finally, we get the actual orbital used in XeF 4 development, and it results in sp 2 d 2 hybridization.
NCERT Solutions for Class 11 Chemistry Chapter 4 ... Answer: Bonding molecular orbital has lower energy and higher stability. Question 19. Define antibonding molecular orbital. Answer: The molecular orbital formed by the subtractive effect of the electron waves of the combining atomic orbitals, is …
(PDF) Bonding and Electronic Structure of XeF 3 | Kim ... Download. Bonding and Electronic Structure of XeF 3. Kim Lobring. Subscriber access provided by NATIONAL CHUNG CHENG UNIV Article Bonding and Electronic Structure of XeF 3- Ian H. Krouse, Changtong Hao, Catherine E. Check, Kim C. Lobring, Lee S. Sunderlin, and Paul G. Wenthold J. Am. Chem. Soc., 2007, 129 (4), 846-852• DOI: 10.1021/ja065038b ...
Draw a molecular orbital diagram for XeF, and use the aufbau ... Draw a molecular orbital diagram for XeF, and use the aufbau principle to put in the appropriate number of electrons. Is XeF+ likely to be more stable than XeF?4 answers · Top answer: Okay. SF six it has a highlight on infinity and becomes the more stables when I pick ...
Hybridization of XeF2 - Hybridization of Xe in Xenon ... The central atom Xe has 2 bond pair and 3 lone pairs around it. During hybridization, Xenon will form two sigma bonds with two fluorine atoms. There are three hybrid orbitals that contain the lone pairs and they do not form any bonds. XeF2 Molecular Geometry And Bond Angles XeF2 molecular geometry is linear.
Xef2 Lewis Structure, Polarity, Hybridization and shape Xef2 Lewis Structure, Polarity, Hybridization and shape. XeF2 is an abbreviation for the chemical compound Xenon Difluoride. It is a powerful fluorinating as well as an oxidizing agent. Apart from XeF2, there are other Xenon compounds such as XeF4 ( Xenon Tetrafluoride) and XeF6 ( Xenon Hexafluoride). Out of these compounds, XeF2 is the most ...
Photoionization from the Xe 4d orbitals of XeF 2 (Journal ... The Xe 3d-ϵf continuum shape resonances dominate the photoionization cross sections of both the atom and molecule, but prominent resonances appear in the XeF{sub 2} cross section due to nominal excitation of Xe 3d and F 1s electrons to the lowest unoccupied molecular orbital (LUMO), a delocalized anti-bonding MO.
![Answered: ' B 2u Dah [F4 ] (5 p orbitals) (2 p… | bartleby](https://prod-qna-question-images.s3.amazonaws.com/answer/760ff081-3d3a-471f-8703-be5f4cb7a234/f4cb7eee-4629-4f6d-b6a1-6a3764fdd1dc/4lmqhk8.png)

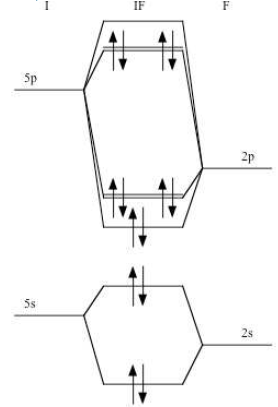


![PDF] Molecular Structure of XeF6. II. Internal Motion and ...](https://d3i71xaburhd42.cloudfront.net/07b520c642d1485745e4d6aecca8960af8d73974/2-Figure1-1.png)




![Answered: ' B 2u Dah [F4 ] (5 p orbitals) (2 p… | bartleby](https://prod-qna-question-images.s3.amazonaws.com/question/760ff081-3d3a-471f-8703-be5f4cb7a234/562e8086-86b2-4e45-81de-bb85d6ee44f7/7peldhj.jpeg)












0 Response to "42 xef molecular orbital diagram"
Post a Comment