39 carbon electron distribution diagram
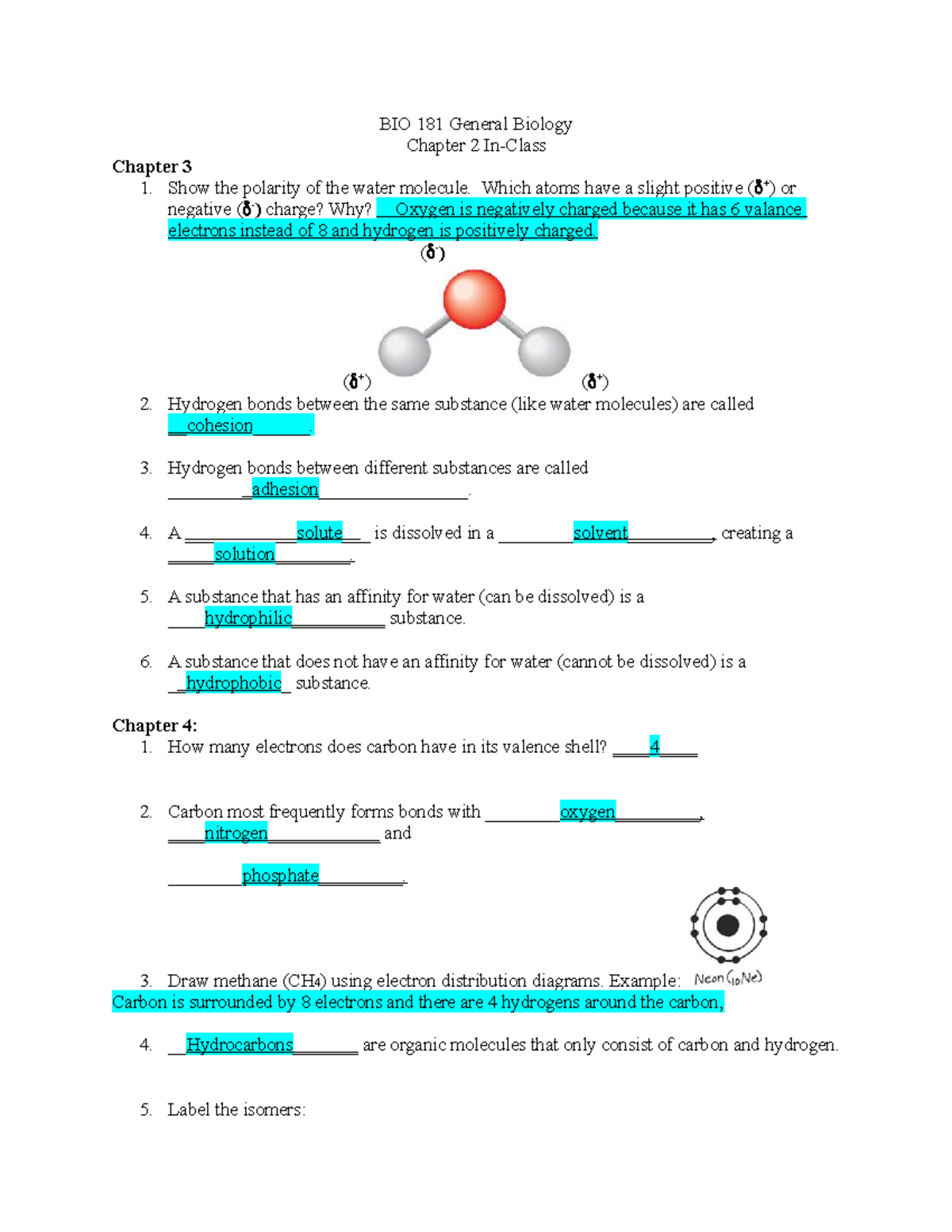
Chapter_3 - Name: Chapter 3 Active Reading Guide Carbon ... Name: Chapter 3 Active Reading Guide Carbon and the Molecular Diversity of Life Section 1 1. Make an electron distribution diagram of carbon. It is essential that you know the answers to these questions: How many valence electrons does carbon have? _4____ How many bonds can carbon form? __4___ What type of bonds does it form with other elements? covalent bonds 2. Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
STRUCTURE OF CARBON MONOXIDE - Ohio State University The low dipole moment and the formation of complexes of CO are explained as due to the presence of a lone pair of electrons on the remote side of the carbon atom. The CO molecule is shown to have a triple-bond structure (two Π and one σ bonding orbitals). Description:

Carbon electron distribution diagram
Chapter 2 BIO mastering biology Flashcards | Quizlet A) The figure contains electron distribution diagram of an atom. It has 2 electrons in the inner shell and 1 electron in the outer shell. B) The figure contains electron distribution diagram of an atom. It has 2 electrons in the inner shell and 8 electrons in the outer shell. C) The figure contains electron distribution diagram of an atom. How to draw electron configuration diagrams | Feature ... An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or 'shells', around the central nucleus. The Electronic Structures of Carbon Monoxide and Carbon ... The electronic structures of carbon monoxide and carbon dioxide BY W. MOFFITT, New College, Oxford (Communicated by F. E. Simon, F.R.S.-Received 6 August 1948) By means of the self-consistent LCAO method, electronic structures are assigned to the ground states of carbon monoxide and carbon dioxide, to their ions and to their more im-
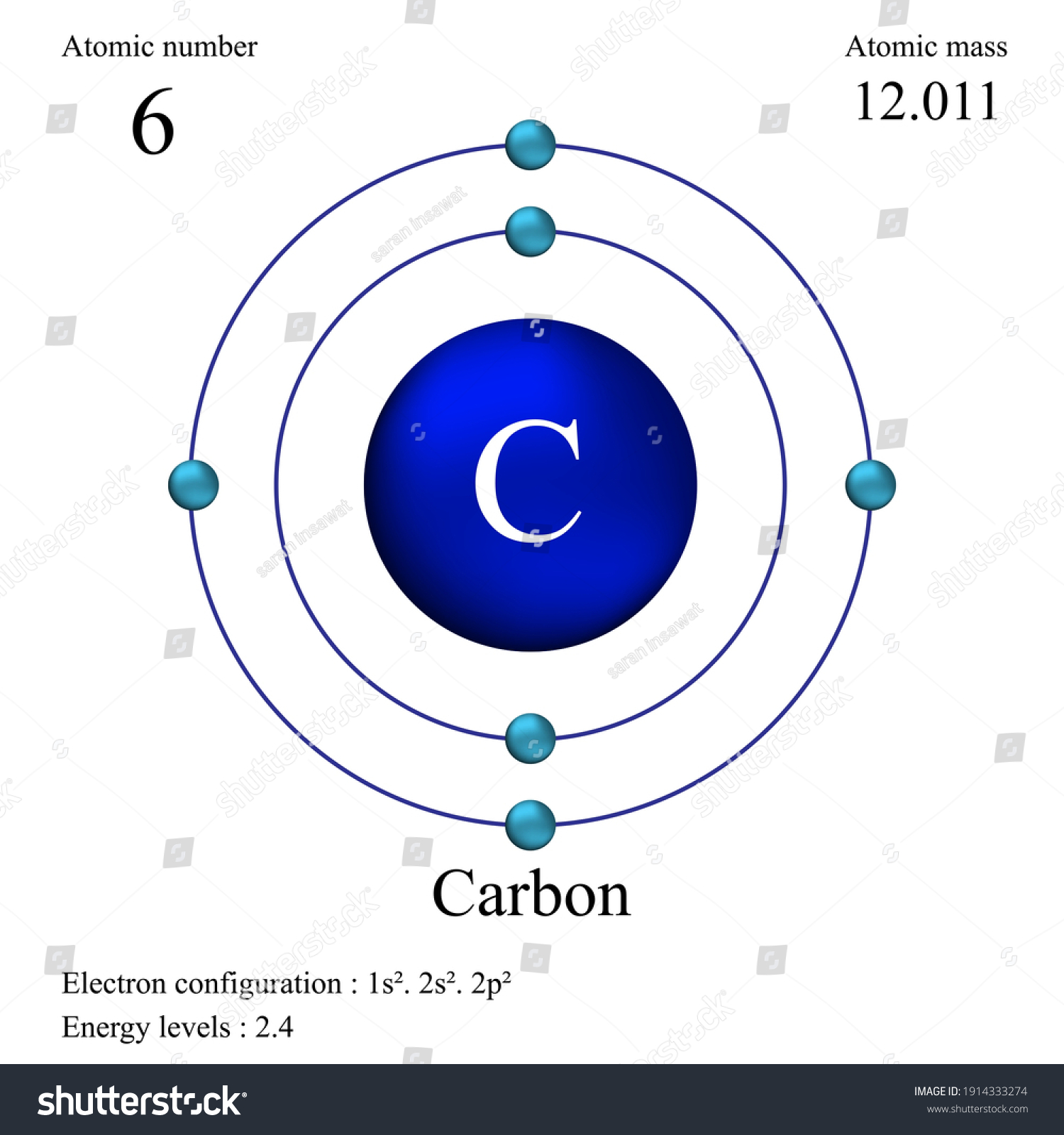
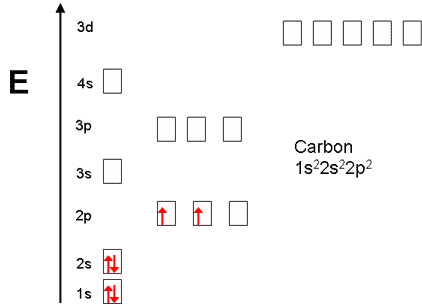
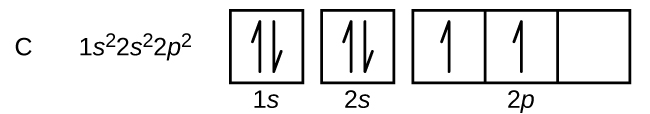
Carbon electron distribution diagram. What is the distribution of electrons in carbon and sodium ... The number of the electron in carbon is 6, and the electronic configuration of carbon can be written as, C (Z = 6) = 1s2 2s2 2p2 The first two electrons are placed in the 1s orbital. The 1s orbital can accommodate two electrons. The next 2 electrons of carbon go in the 2s orbital. The next two electrons will go in the 2p orbital. Carbon Bohr Model - How to draw Bohr diagram for Carbon(C) atom Here, we will draw the Bohr diagram of the Carbon atom with some simple steps. Steps to draw the Bohr Model of Carbon atom 1. Find the number of protons, electrons, and neutrons in the Carbon atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Electron Dot Diagram For Methane - schematron.org Electron Dot Diagram For Methane. Well Carbon only has 4 valence electron, so it can bond at all four point. Hydrogen only has one valence electron and can only share one. Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) . Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown. Carbon Monoxide Molecular Orbital Diagram Explanation There are 4 electrons in the outer shell of carbon and 6.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
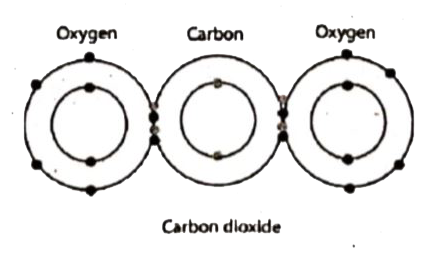
Draw the electron distribution diagram for the formation ... The electron distribution diagram for the formation of carbon dioxide oxide (CO₂) molecule is shown below. Explanation : Electron distribution diagram is also known as Lewis-dot structure. Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. PDF Elements & Compounds 13Al Carbon 6C Silicon 14Si Nitrogen 7N Phosphorus 15P Oxygen 8O Sulfur 16S Fluorine 9F Chlorine 17Cl Neon 10Ne Argon 18Ar Helium 2He 2 He Mass number 4.00 Atomic number Element symbol Electron distribution diagram Fig. 2.9:Electrons are distributed in shells of orbitals. Each orbital contains a maximum of two electrons. Electron Configuration for Carbon (C) - UMD How to Write the Electron Configuration for Carbon Carbon is the sixth element with a total of 6 electrons. In writing the electron configuration for carbon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for C goes in the 2s orbital. The remaining two electrons will go in the 2p orbital. What is the electron dot diagram for carbon? | Socratic Oct 19, 2015 · Explanation: The electron dot diagram of an element or a molecule is called Lewis structure; it features the distribution of valence electrons around elements. Carbon has four valence electrons and therefore, they are drawn on the four sides of a carbon atom as represented in the figures below. Answer link
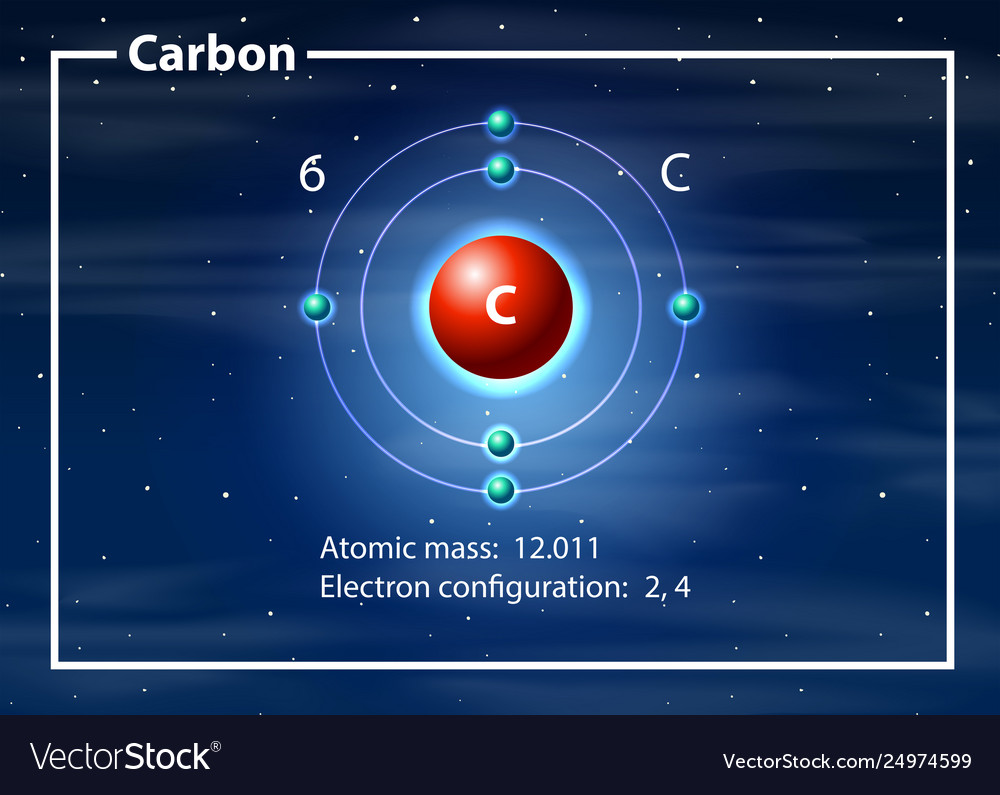
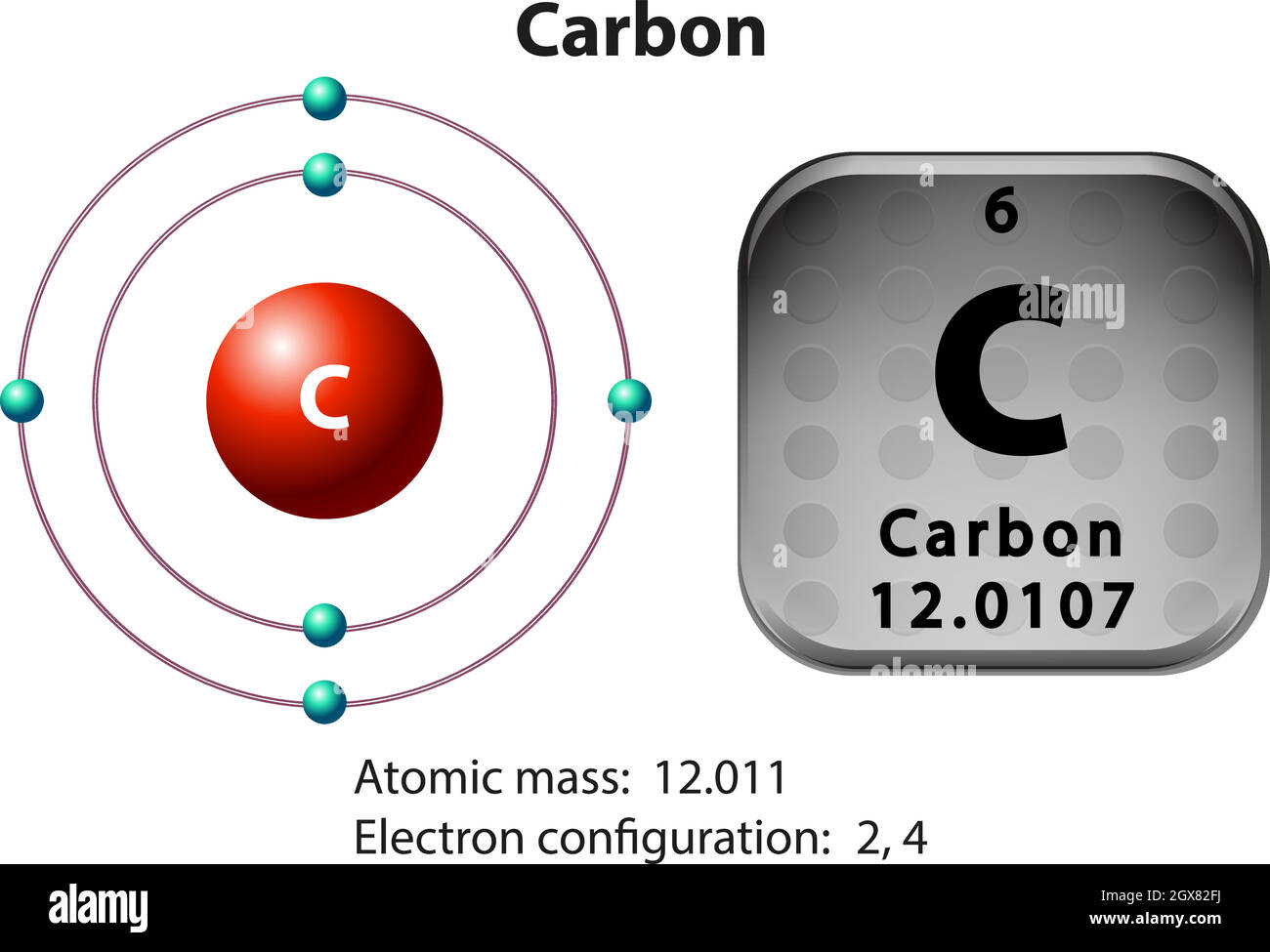
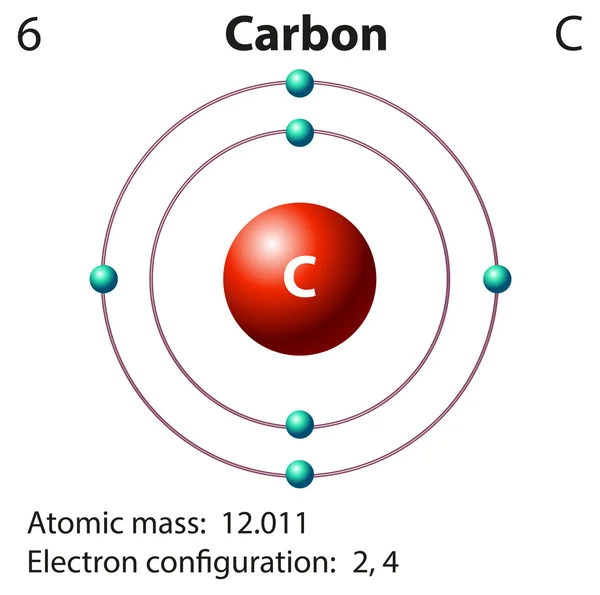
Write the Distribution of Electrons in Carbon and Sodium Atom? The distribution of electrons in the carbon atom is as follows: In the first orbit or K-shell = 2 electrons In the second orbit or L-shell = 4 electrons Or, we can write the distribution of electrons in a carbon atom as 2, 4. Sodium The atomic number of sodium is 11 and the electronic configuration of sodium is, Na (Z = 11) = 1s 2 2s 2 2p 6 3s 1 Draw the electron distribution diagram for the formation ... Draw the electron distribution diagram for the formation of Carbon di oxide (CO2 ) molecule. 0 Tamil Nadu Board of Secondary Education SSLC (English Medium) Class 9th Solved 2. Draw an electron-distribution diagram of a ... Draw an electron-distribution diagram of a carbon atom Show the correct numbers, and approximate locations, of all of this atom's subatomic particles. 2a. Use the following information for your drawing: Atomic number = 6 Mass number - 12 . This carbon atom is neutral 2b. PDF Chapter 4: Carbon and the Molecular Diversity of Life 3. Make an electron distribution diagram of carbon. ! Carbon has 4 valence electrons, can bond to 4 items, and typically forms covalent bonds with other elements. 4. Carbon chains form skeletons. List here the types of skeletons that can be formed. Carbon skeletons vary in length. The skeleton may have double bonds, which can vary in location.
Chapter 4: Carbon and the Molecular Diversity of Life ... Make an electron distribution diagram of carbon. It is essential that you know the answers to these questions: How many valence electrons does carbon have? 4 How many bonds can carbon form? 4 What type of bonds does it form with other elements? Covalent bonds Carbon chaines form skeletons. List the ypes of skeletons that can be formed.
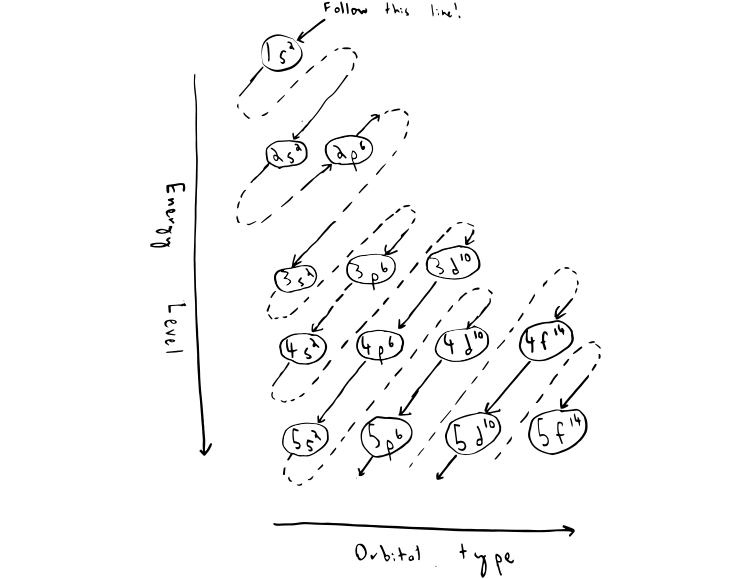
Electron configuration for Carbon (element 6). Orbital diagram Melting point: 3550 ℃. Density: 3.51 g/cm 3 . Electronic configuration of the Carbon atom: 1s 2 2s 2 2p 2. Reduced electronic configuration C: [He] 2s 2 2p 2. Below is the electronic diagram of the Carbon atom Distribution of electrons over energy levels in the C atom. 1-st level (K): 2. 2-st level (L): 4.
Carbon monoxide (CO) Molecule Lewis Structure Carbon is a group VIA element in the periodic table and contains six electrons in its last shell. Now, we know how many electrons are there in valence shells of carbon and oxygen atoms. valence electrons given by carbon atoms = 4 * 1 = 4 valence electrons given by oxygen atoms = 6 * 1 = 6 Total valence electrons = 10 Total valence electrons pairs
Carbon dioxide (CO2) lewis dot structure, molecular ... The electron geometry of CO2 is also linear. In the CO2 lewis structure, there is a total of 4 lone pairs present. Two lone pairs on each oxygen atom. The bond angle of CO2 is 180º. Since it is linear in shape with an arrangement like that O=C=O. Two types of hybridization in CO2 - Sp, and Sp2.
Carbon(C) electron configuration and orbital diagram Carbon (C) excited state electron configuration and orbital diagram When the carbon atom is excited, then the carbon atom absorbs energy. As a result, an electron in the 2s orbital jumps to the 2p z sub-orbital. Therefore, the electron configuration of carbon (C*) in excited state will be 1s 2 2s 1 2p x1 2p y1 2p z1.
Orbital Diagram For Carbon (C) | Carbon Electron Configuration Electron Configuration of Carbon One major point before we come to the carbon electron dot diagram is that it is also called a Lewis dot diagram. Therefore the users don't need to get confused between these two as both are the same. The reason behind the name given to Lewis is that it was firstly used by Gilbert N. Lewis.
Electron Configuration Diagrams | Properties of Matter ... Electron Configuration Diagrams | Properties of Matter | Chemistry | FuseSchoolLearn the basics about Drawing electron configuration diagrams. Find out more ...
Electron Microscopy of Carbon Nanotube Composites - NIST Description Three-dimensional view of carbon nanostructures grown on the surface of a glass fiber (marked in orange). The image was created by taking pictures at multiple angles using energy filtered transmission electron microscopy.
CCl4 Lewis Structure, Molecular Geometry, Hybridization ... CCl4 is also named carbon chloride, methane tetrachloride, benziform, and more. The liquid is not soluble in water and is non-combustible. The boiling point of CCl4 is 76.8 degrees Celcius and its melting point is -23.0 degrees Celcius. CCl4 will release toxic fumes like carbon monoxide. if it is led to decomposition.
Write the distribution of electrons in carbon ... - SaralStudy The distribution of electrons in Carbon and Sodium atom : Carbon : Atomic no. of Carbon = 6 no. of protons = 6 no. of protons = no. of electrons Distritribution of electron = K L 2 4 Sodium : Atomic no. of Sodium = 11 no. of protons = 11 no. of protons = no. of electrons Distritribution of electron = K L M
Atom Diagrams: Electron Configurations of the Elements For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left.
The Electronic Structures of Carbon Monoxide and Carbon ... The electronic structures of carbon monoxide and carbon dioxide BY W. MOFFITT, New College, Oxford (Communicated by F. E. Simon, F.R.S.-Received 6 August 1948) By means of the self-consistent LCAO method, electronic structures are assigned to the ground states of carbon monoxide and carbon dioxide, to their ions and to their more im-
How to draw electron configuration diagrams | Feature ... An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or 'shells', around the central nucleus.
Chapter 2 BIO mastering biology Flashcards | Quizlet A) The figure contains electron distribution diagram of an atom. It has 2 electrons in the inner shell and 1 electron in the outer shell. B) The figure contains electron distribution diagram of an atom. It has 2 electrons in the inner shell and 8 electrons in the outer shell. C) The figure contains electron distribution diagram of an atom.






















:max_bytes(150000):strip_icc()/sodiumatom-58b602715f9b5860464c7a22.jpg)



0 Response to "39 carbon electron distribution diagram"
Post a Comment