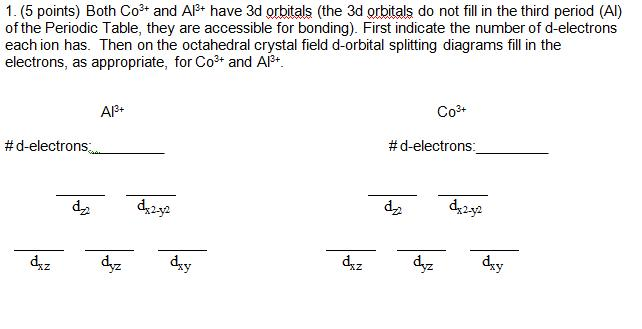
37 co3+ orbital diagram
The remaining one is a non-bonding orbital but doubly field, which denotes the lone pair of phosphorus. Given below is the MO diagram of PF3 taking reference to which you can easily draw for PCl3. A MO diagram helps us to know about the bonding, bond order, bond angle, and bond length of any compound. Below is the video snippet attached for the ... SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. SF4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs. This compound is generally identified as being a colorless gas. The molecular weight of this compound is calculated to be 108.6 g/mol. SF4's boiling and melting points are -38 ...
A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao molecular orbital method in particular.
Co3+ orbital diagram
O. diagram for [Co(NH3)6]3+ Dr. Mithil Fal Desai Shree Mallikarjun and Shri Chetan Manju Desai College Canacona Goa 2. t* 1u a1g t2g, eg a1g, t1u, eg a1g t1u a* 1g e* g eg t1u Δo t2g Metal (Ti3+)orbitals Co3+→[Ar] 3d6, 4s0 6e- Ligand group (NH3) orbitals 6 x 2 = 12 e- σ [Co(NH3)6]3+ molecular orbitals M. O. diagram for [Ti(H2O)6]3+ complex ... Carbonate mineralization is reasonably well-understood in the Ca-CO2-H2O system but continuously poses difficulties to grasp when Mg is present. One of the outstanding questions is the lack of success in dolomite MgCa(CO3)2 crystallization at atmospheric conditions. The conventional view holds that hydration retards the reactivity of Mg2+ and is supported by solvation shell chemistry. MO Diagram of H2CO3. The sigma bonds between C and O atoms are formed by the 2sp2 orbital of C and O atoms. It results in sigma bonding and antibonding orbitals. The sigma bond between oxygen and hydrogen use 1s orbital of hydrogen and 2sp3 orbitals of oxygen. It forms a sigma bonding and antibonding orbital.
Co3+ orbital diagram. electronic configuration” of elements. spdf. Notation orbital box diagram ... ions: Sc+3, Zn+2,Co2+ and Co3+ . Distinguish if.11 pages Does co3 have a pi bond? - Carbonate ion has one carbon atom, three oxygen atoms and -2 charge. One unhybridized 2p orbital of carbon will laterally overlap with one unhybridized 2p orbital of oxygen to form pi-bond. Thus, there is a presence of one double bond in the carbonate ion. Does ozone have delocalized pi bonds? A simplified molecular orbital diagram for an octahedral transition metal complex showing σ−and π−interactions only. Class I : In class I complexes, the Δ o splitting is small and often applies to 3d metals and σ ligands at lower end of the spectrochemical series. ClO3- Lewis Structure, Molecular Geometry, Hybridization & Shape. May 3, 2021. Posted by Priyanka. 17 Apr. The chemical formula ClO 3- represents Chlorate ion. Chlorine can reach oxidation states of +1, +3, +5 and +7. In this case, as seen in the figure, Chlorates exist in a +5 oxidation state. With an abundance of oxidizing elements, the ...
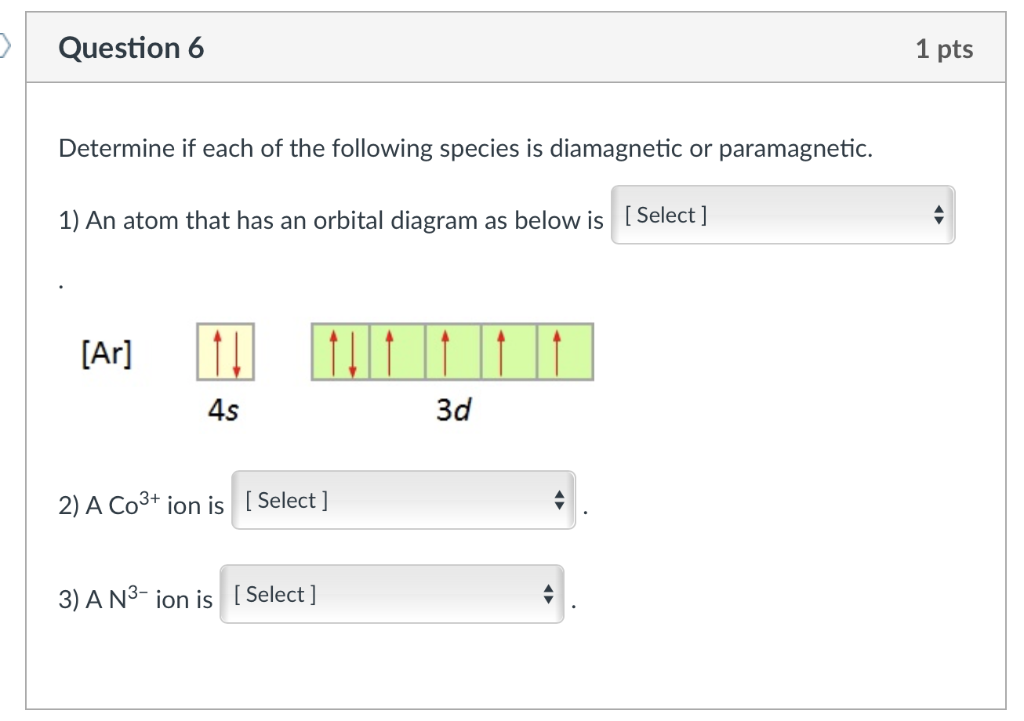
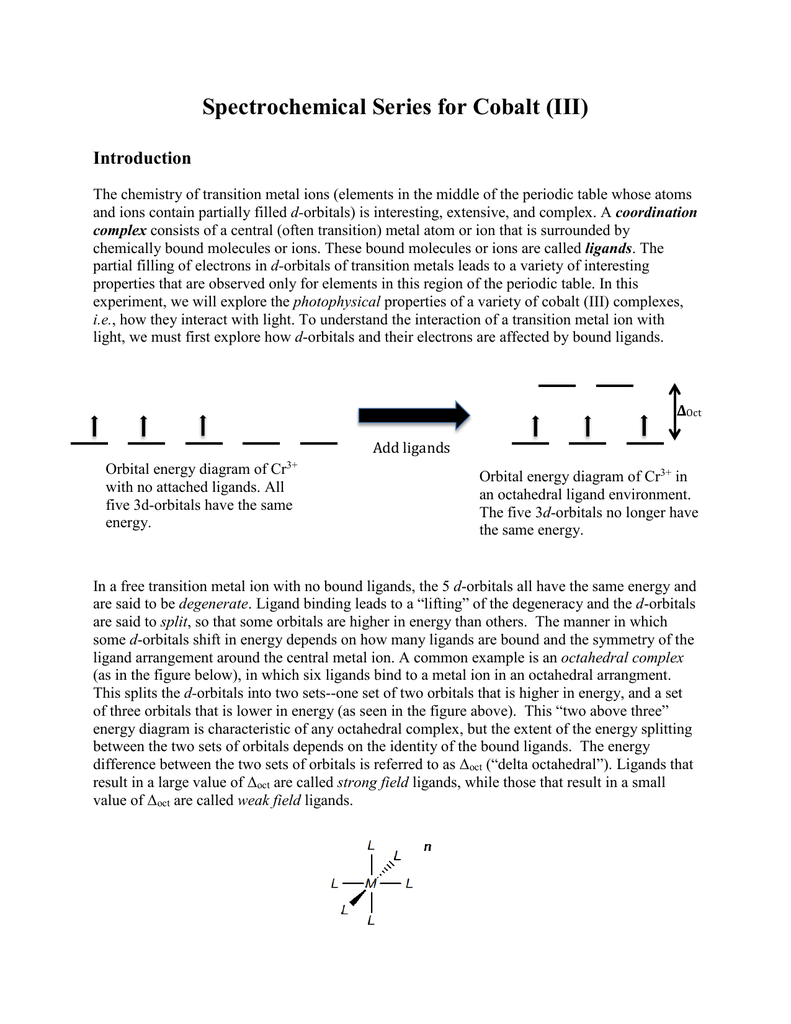
The metals (Lewis acids) have d orbitals that are partially filled ... depending on the amount of orbital overlap with the ligand. ... Co3+ (d6).23 pages NO3 Lewis Structure, Molecular Geometry, and Hybridization. NO3 is a polyatomic ion with a negative charge. So, it is also referred to by the name of nitrogen oxoanion. The compound has its chemical name as nitrate formed after nitric acid looses a proton from it. Nitrate is an important source of nitrogen and oxygen. By Hund's rule, the electron configuration of carbon, which is 1s2 2s2 2p2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Which of the following has maximum number of unpaired electrons fe3+ Fe2+ Co2+ Co3 ... A gaseous Co3+ ion in the ground state has four unpaired electrons. Given this, how many unpaired electrons are in an atom of carbon in its ground state? By Hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2 , is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state ...
Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. Now carbon monoxides mo diagram is. Jmol models of wavefunctions calculated at the rhf321g level. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao molecular orbital method in particular. The CFT diagram for tetrahedral complexes has d x 2 −y 2 and d z 2 orbitals equally low in energy because they are between the ligand axis and experience little repulsion. In square planar molecular geometry, a central atom is surrounded by constituent atoms, which form the corners of a square on the same plane. CHEM 103 Exam 1-6 Final - Complete Solutions, Graded A Exam 1-6 Question 1 1. Convert 1005.3 to exponential form and explain your answer. 2. Convert 4.87 x 10-6 to ordinary form and explain your answer. Question 2 Using the following information, do the conversions shown below, showing all work: 1 ft = 12 inches 1 pound = 16 oz 1 gallon = 4 quarts 1 mile = 5280 feet 1 ton = 2000 pounds 1 quart ...
Transition Metals with an Oxidation State. In the ground state, the electron configuration of the transition metals follows the format, ns 2 nd x.As for the electron configuration for transition metals that are charged (i.e. Cu +), the electrons from the s orbital will be moved to the d-orbital to form either ns 0 nd x or ns 1 nd x.. It is helpful to first write down the electron configuration ...
SO3 Molecular Geometry, Lewis Structure, and Polarity Explained. SO3 stands for Sulfur Trioxide. This is one of the most pollutant chemical compounds in the gaseous form. It is also a primary agent in the acid rain. The main use of this component is to make sulfuric acid for industrial purposes.
26 Jan 2021 — The Cobalt Electron Configuration (Co) with Orbital Diagram and Cobalt valence electrons have been provided here with the pictures.Missing: co3+ | Must include: co3+
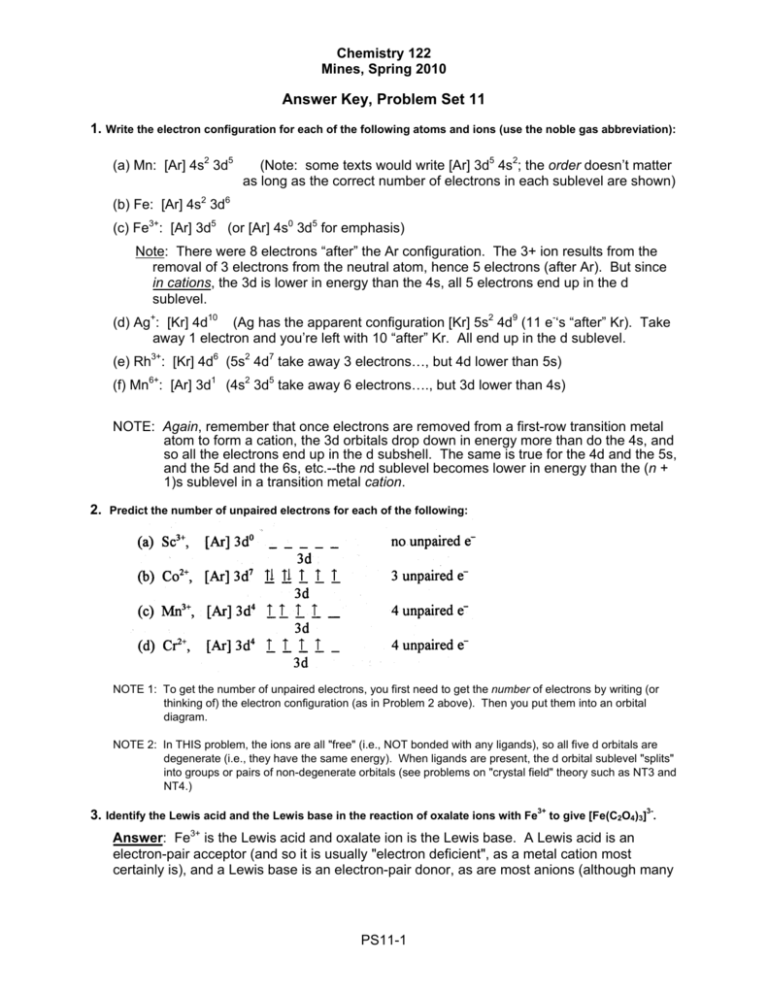
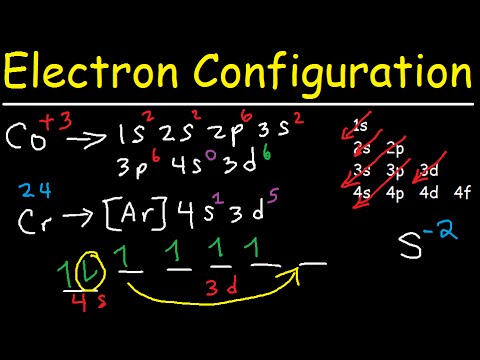
3 Jan 2016 · 1 answerThe electron configuration of Co3+ is [Ar]4s3d5 . Co is in Period 4 of the Periodic Table, and Ar is the preceding noble gas.
Here are electron configurations of Co, Co+, Co2+ and Co3+: Co [Ar] 3d7 4s2 ... Why doesn't the electrons in the s-orbital move to the 3D-orbital so it can ...9 answers · 11 votes: Transition metals, when losing electrons, first lose s electrons and then d electrons. ...
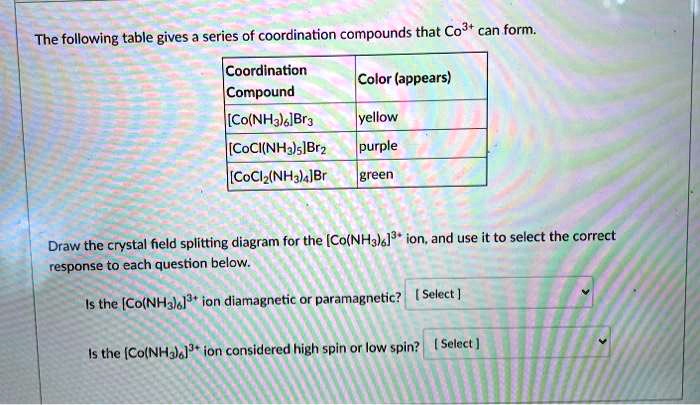
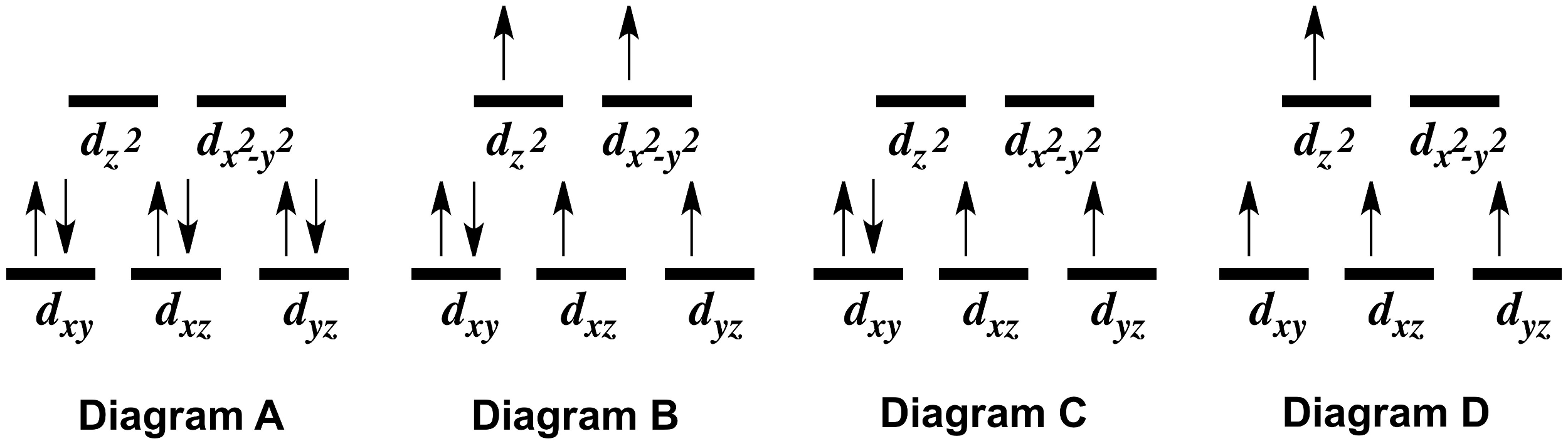
Solved The Following Table Gives Series Of Coordination Compounds That Co3 Can Form Coordination Compound I Co Nha Ibra Color Appears Yellow Coci Nhalsibrz I Coch Nh Ibr Purple Green Draw The Crystal Field Splitting Diagram For The Co Nh3la Ion
Craftsman 917296030 rear - tine tiller parts - manufacturer-approved parts for a proper fit every time! We also have installation guides, diagram s and manuals to help you along the way! Use our part lists, interactive diagram s, accessories and expert repair advice to make your repairs easy. 877-346-4814.
Carbon trioxide | CO3 | CID 520883 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. COVID-19 Information. Public health information (CDC) Research information (NIH) SARS-CoV-2 data (NCBI) Prevention and ...
A molecular orbital diagram of .NO shows it to have a bond order of 2.5 and one unpaired electron in a π2p* antibonding orbital (hence the notation .NO). ... When it reacts with CO2, it can form either NO3- and CO2 (65%) or CO3.- and .NO2 (35%). These latter products can oxidize organic molecules in one electron steps. Peroxynitrite can also ...
Quantum numbers. There are four quantum numbers n, l, m l, and m s.The principal quantum number n is a positive integer (1,2,3,4) and it represents the energy of the orbital.The angular momentum quantum number l, is from 0 to n - 1. The l values of 0, 1, 2, and 3 correspond to the s, p, d and f orbitals, respectively. The magnetic quantum number m l ranges from -l to +l.
Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from solving the Schrodinger's equation for Bohr's hydrogen atom. Hence, many of the rules that we use to describe the electron's ...
One unhybridized 2p orbital of carbon will laterally overlap with one unhybridized 2p orbital of oxygen to form pi-bond. Thus, there is a presence of one double bond in the carbonate ion. - Therefore, the carbon atom in carbonate ion, CO2−3 is sp2 hybridized.. What is the hybridization of carbon in CO3 2 −?,
For the carbonate ion, \(\ce{CO3^2-}\), draw all of the resonance structures. Identify which orbitals overlap to create each bond. ... Draw the orbital diagram for carbon in CO 2 showing how many carbon atom electrons are in each orbital.
Answer (1 of 2): In strong field delta0 will be more. Co =3d74s2 Co +3= 3d6 CN- is considered as strong ligand, hence all 6 electrons will be filled in t2g orbitals. All paired. Diamagnetic, inner orbital complex, d2sp3. Now in case of Br- is considered as weak ligand hence all 6 electrons wi...
CO32- Molecular Orbital (MO) Diagram What is MO theory? Molecular Orbital Theory is a concept of quantum mechanics that is used to decipher the chemical bonding nature inside different molecular structures. This is a complex yet useful tool that helps in sketching MO diagrams for better understanding.
(SHOW WORK, DRAW THE ORBITAL DIAGRAM) O 0 0 1 04 O 3 7 O 6 O 5 02 QUEST ION 15 Which of the following is diamagnetic? (SHOW WORK, DRAW THE NOBLE GAS ORBITAL DIAGRAM S) O 37Rb O 15P 13A1 0 24Cr O 48Cd QUEST ION 16 Answer Draw the orbital diagram of the element. Explain the two rules applied in arranging the electrons in the orbital s. 8Q: 2p ...
The p orbital can hold up to six electrons. The atomic number of sodium is 11. And like it was said before. The Electron Configuration of Sodium. 1s 2 2s 1 He2s 1. This video shows how to draw the orbital diagram of Sodium Na. Favorite Answer Sodium 1s2 2s2 2p6 3s1 Iron 1s2 2s2 2p6 3s2 3p6 3d6 4s2 I cant give colbalt as it doesnt exist.
MO Diagram of H2CO3. The sigma bonds between C and O atoms are formed by the 2sp2 orbital of C and O atoms. It results in sigma bonding and antibonding orbitals. The sigma bond between oxygen and hydrogen use 1s orbital of hydrogen and 2sp3 orbitals of oxygen. It forms a sigma bonding and antibonding orbital.
Carbonate mineralization is reasonably well-understood in the Ca-CO2-H2O system but continuously poses difficulties to grasp when Mg is present. One of the outstanding questions is the lack of success in dolomite MgCa(CO3)2 crystallization at atmospheric conditions. The conventional view holds that hydration retards the reactivity of Mg2+ and is supported by solvation shell chemistry.
O. diagram for [Co(NH3)6]3+ Dr. Mithil Fal Desai Shree Mallikarjun and Shri Chetan Manju Desai College Canacona Goa 2. t* 1u a1g t2g, eg a1g, t1u, eg a1g t1u a* 1g e* g eg t1u Δo t2g Metal (Ti3+)orbitals Co3+→[Ar] 3d6, 4s0 6e- Ligand group (NH3) orbitals 6 x 2 = 12 e- σ [Co(NH3)6]3+ molecular orbitals M. O. diagram for [Ti(H2O)6]3+ complex ...













0 Response to "37 co3+ orbital diagram"
Post a Comment