39 co2+ orbital diagram
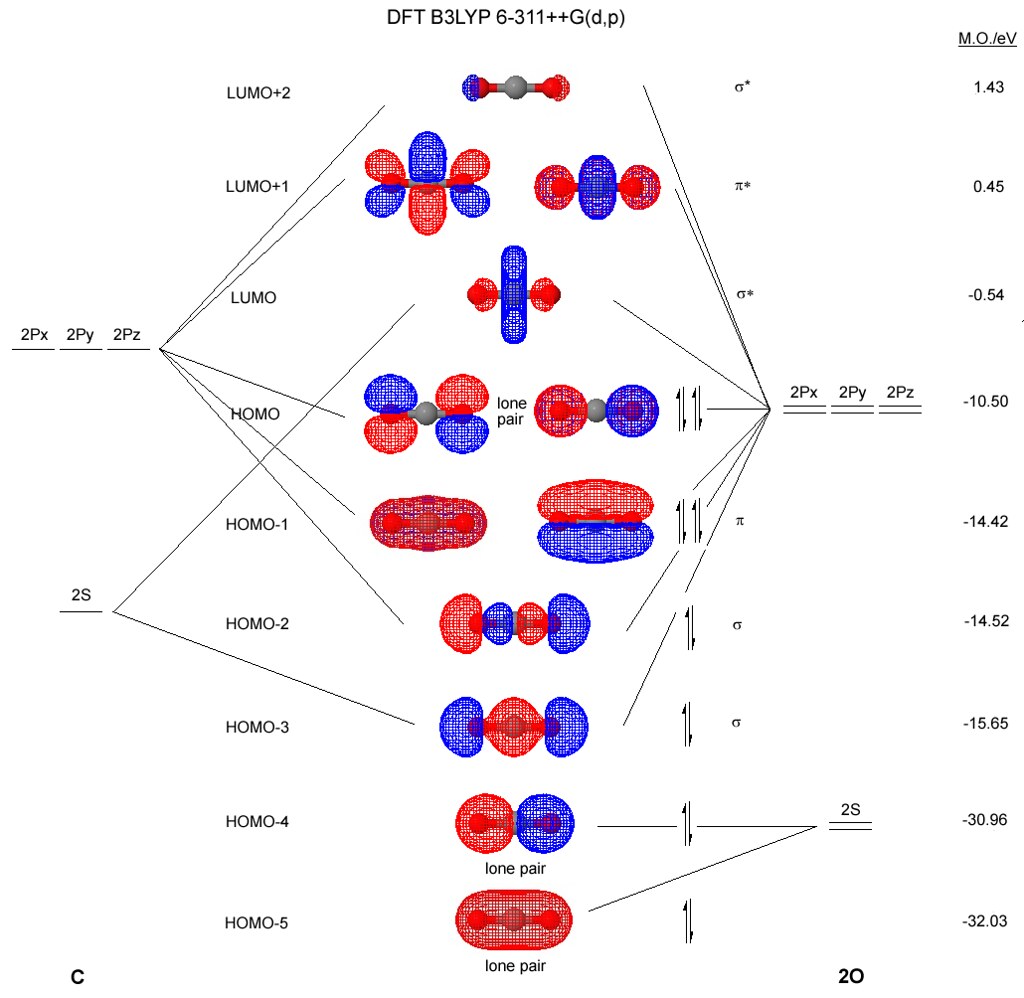
See also: Molecular orbital diagram § Carbon dioxide. This produces a positive feedback for changes induced by other processes such as orbital cycles.[101] Five hundred million years ago the "Vostok ice core: climatic response to CO2 and orbital forcing changes over the last climatic cycle". A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
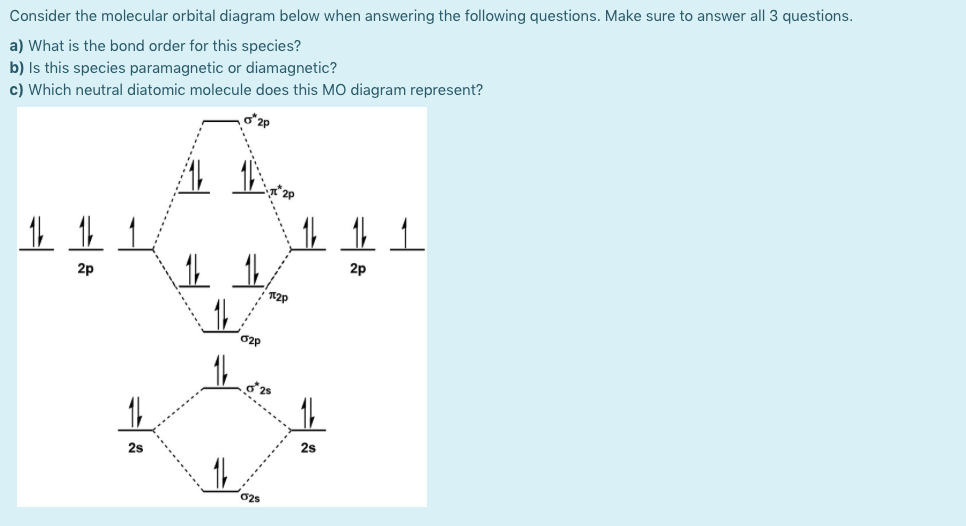
Here we have a molecular orbital diagram for the CO molecule. So again, it's drawn in the familiar pattern. You have the, here on this side you would have the energy, so the energy is going up there.

Co2+ orbital diagram
co molecular orbital diagram Molecular electron configuration for o2 σ2σ2σ2π4π2 we can also calculate the oo bond order. Co orbital diagram. • Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. The carbon dioxide MO diagram is based on a C atom and an O--O ligand fragment. Carbon has 2S and 2Px,y,z orbitals and the O--O fragment has 2S and 2Px,y,z orbitals that are involved in the formation of molecular orbitals. ... The 2πg orbiatls are nonbonding because the C 2Px,y atomic... Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+.
Co2+ orbital diagram. Go Now All travel. Co2+ Orbital Diagram. Details: Apr 06, 2019 · Watch the video solution for the question: Draw the orbital diagram for ion Co 2+.. . can be accommodated in the metal d orbitals. • d0 ions •d7 ions - Fe1+, Ru1+, Co2+, Rh2+, Ni3+, etc. . σ-ML4 Tetrahedral MO Diagram e. Answer to... Similarly, with Be2+ as well, there are 2(4) - 1 = 7 total electrons if you're filling out a complete MO diagram. 10. Remember, valence electrons are those which do not represent a noble-gas-like-state. the 1s Orbital is full (2 electrons), so the Be2+ configuration is the same as Helium. the 2s orbital in... Crystal field d orbital splitting diagrams for common geometries. The above treatment considers the ligands in an octahedral geometry (i.e., with the Co2+(H2O)6 is assumed to be the chromophore. Their provocative conclusion is that the aqueous absorption spectrum is due to a strongly-absorbing... Construct the orbital diagram of each atom or ion. Part b draw the orbital diagram for ion co2. Complex Ions...
He2+. Lewis Structure: Molecular Orbital Energy Diagram. Total # of bonding electrons. # of Sigma Bonds. Should this molecule exist? MO electron configuration: CO-. Lewis Structure: Molecular Orbital Energy Diagram. 32 Co Orbital Diagram - Wiring Diagram Info. 8 hours ago So you have the carbon two s orbital and you have the carbon two p orbitals. Just Now Carbon Monoxide Molecular Orbital Diagram Explanation. generic s-p valence MO diagram for carbon monoxide CO chain one can reasonably... Carbon dioxide (CO2), molecule is triatomic and linear like Beryllium di hydride (BeH2) However, unlike hydrogen as peripheral atoms in BeH2, there are... Examples: (all d7 Co2+ complexes) [Co(H2O)6]2+ looks purple in its salts and in concentrated solution because it absorbs in the green range. d-orbital energy diagrams for high and low spin Co2+ complexes, d7. Rules of thumb
orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y, 2p z, 3s, 3p x, 3p y, 3p z, 4s orbital and two of the 3d orbitals, with only 1 electron occupying each of the other 3d orbitals and these electrons have parallel spin (arrows. pointing in the same direction)... Lecture 6: ML6 molecular orbital energy diagrams incorporating p-acceptor and p-donor ligands. Electron counting revisited and link to spectrochemical series. Conclusive proof of inner sphere mechanism If we use [Co(NH3)5Cl]2+ at start and add Cl*-, Cl*- is not in final product. Splitting d-orbital diagram for [Co(NH3)6]2+ is: t2g6eg1. The complex [Cu(en)3]2+ has an octahedral shape, en is a strong field ligand, but the appropriate crystal field diagram shows that only one configuration is possible irrespective of the strength of the ligand field. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding. in molecules. In the case of CO, the 2s atomic orbital on Oxygen is much lower in energy than the 2s atomic orbital in carbon.
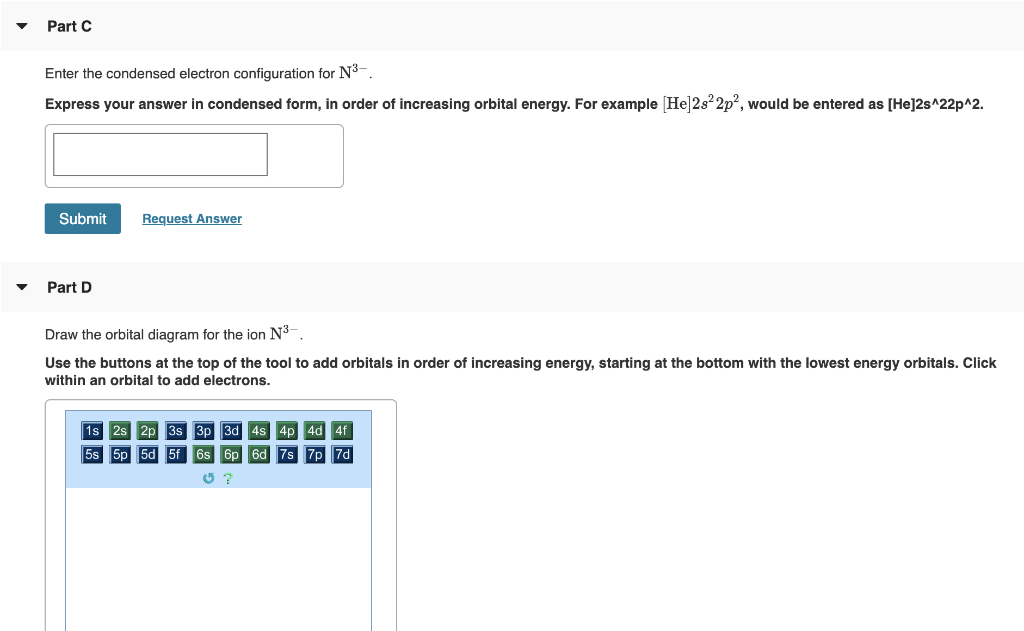
Co2+ Orbital Diagram - Wiring Diagram Pictures. On roundup of the best FAQs on www.schematron.org ▼. Apr 06, 2019 · Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the.
Answer to Write orbital diagram for Co2+. Use the buttons at the top of the tool to add orbitals. Answer to: How to draw molecular orbital diagram for CO2 By signing up, you'll get thousands of step-by-step solutions to your homework questions.SWBAT complete electron orbital diagrams (using...
How do you write the orbital diagram for carbon. The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at...
Related questions. draw the orbital diagram for the ion co2+.
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
Draw The Orbital Diagram For The Ion Co2+. Details: Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at.
By Writing Molecular Orbital Configuration For No Co O2 Molecules Calculate The Bond Order And Also Determine Whether It Is Paramagnetic Or Diamagnetic Socratic
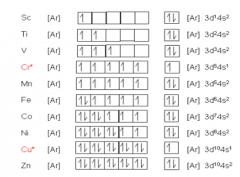
An orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron spin within orbitals. The presenter then goes over the example. Next, the video goes over an ions with a charges S2−, Al3+, and Co2+.
Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams, Chemistry Viva, Chemistry Job Molecular orbital diagram of H2+ (Hydrogen molecule ion)
Origin Of The Ground Kramers Doublets For Co2 3d7 Ions With The Effective Spin 3 2 Versus The Fictitious Spin Springerlink
Molecular Orbital Diagram of Polyatomic CO2 Molecules - Chemical Bonding & Molecular Structures. Molecular Orbital diagram of BN, CN, CN- trclips.com/video/DlIQsEEvicc/video.html ¤Molecular MO DIAGRAM O2+ , O2 2+ ,O2- ,O2 2- (preparation of gate /csir net/uset/set exam ).

Two Orbital Three Electron Stabilizing Interaction For Direct Co2 As3 Bonds Involving Square Planar Coo4 In Bacoas2o5 Semantic Scholar
Orbital Diagram For Nitrogen. Quizlet is the easiest way to study, practise and master what you're learning. Create your own flashcards or choose from millions created by other students.

Addressing Metal Centres In Supramolecular Assemblies Chemical Society Reviews Rsc Publishing Doi 10 1039 B517267p
Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals. Click within an orbital to add electrons.
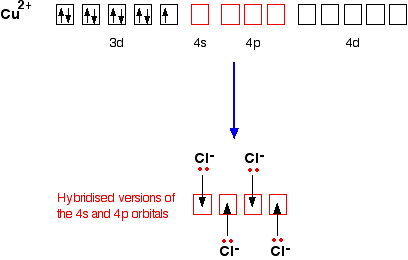
Each Co2+ cation is ferromagnetically aligned with four cation in neighbouring chains and antiferromagnetically aligned with two others. Thus, the dominant exchange mechanism is one that activates the bridge moiety via the spin polarization of a doubly occupied orbital with phenylene...
Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+.
The carbon dioxide MO diagram is based on a C atom and an O--O ligand fragment. Carbon has 2S and 2Px,y,z orbitals and the O--O fragment has 2S and 2Px,y,z orbitals that are involved in the formation of molecular orbitals. ... The 2πg orbiatls are nonbonding because the C 2Px,y atomic...
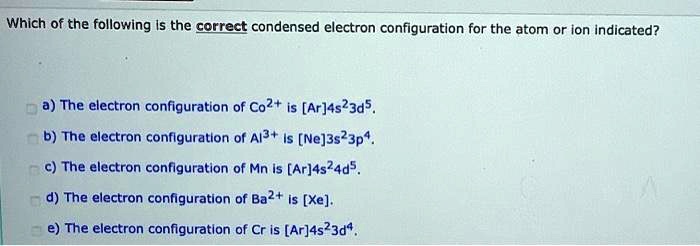
Solved Which Of The Following Is The Correct Condensed Electron Configuration For The Atom Or Ion Indicated A The Electron Configuration Of Co2 Is Ar 4s 3d5 B The Electron Configuration Of Alj Ne 3s 3p4
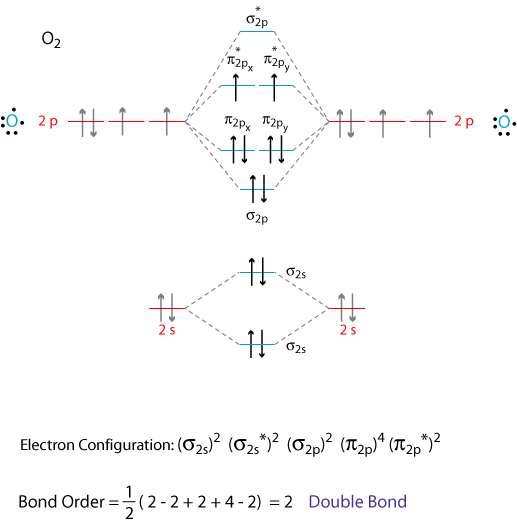
co molecular orbital diagram Molecular electron configuration for o2 σ2σ2σ2π4π2 we can also calculate the oo bond order. Co orbital diagram. • Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable.

Quinoline Based Hydrazone For Colorimetric Detection Of Co2 And Fluorescence Turn On Response Of Zn2 Sciencedirect

Aqueous Solutions Of Co2 Salts Are Pale Pink They Contain The Complex Ion Co H2o 6 2 Addition Of Homeworklib
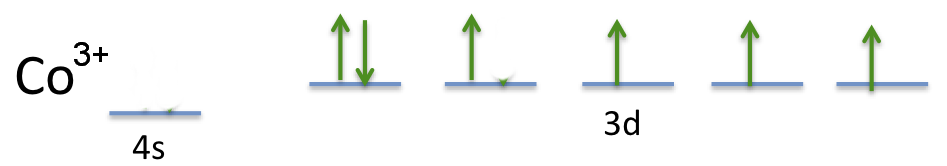
How Many Unpaired Electrons Are In A Gaseous Co 3 Ion In Its Ground State The Answer Is 4 But I Don T Understand How Could Someone Please Explain Thank You Socratic

Solved Rank The Ligands In Order Of Their Increasing Effect On Doct For Co2 Data Co2 Ligand Color Seen Color Absorbed H20 Light Pink Green Cl Azure Blue Orange C2o42 Light Pink Green













0 Response to "39 co2+ orbital diagram"
Post a Comment