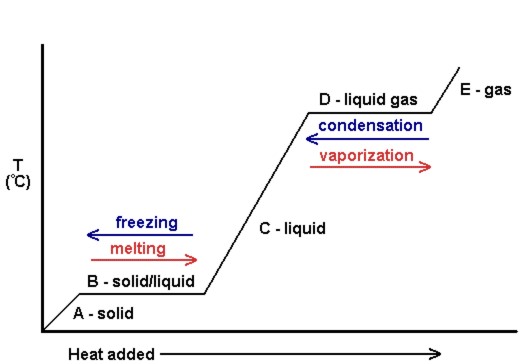
37 in the diagram which letter represents the transition from gas to liquid
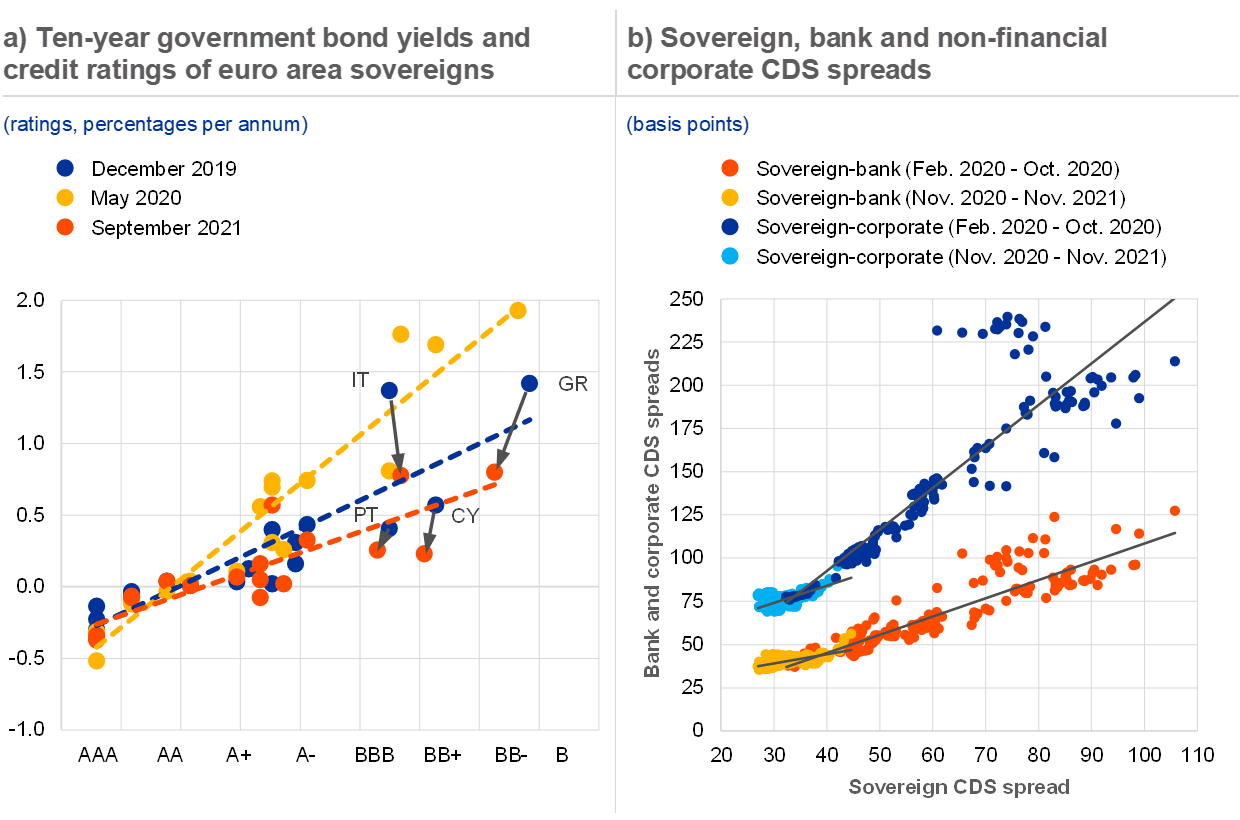
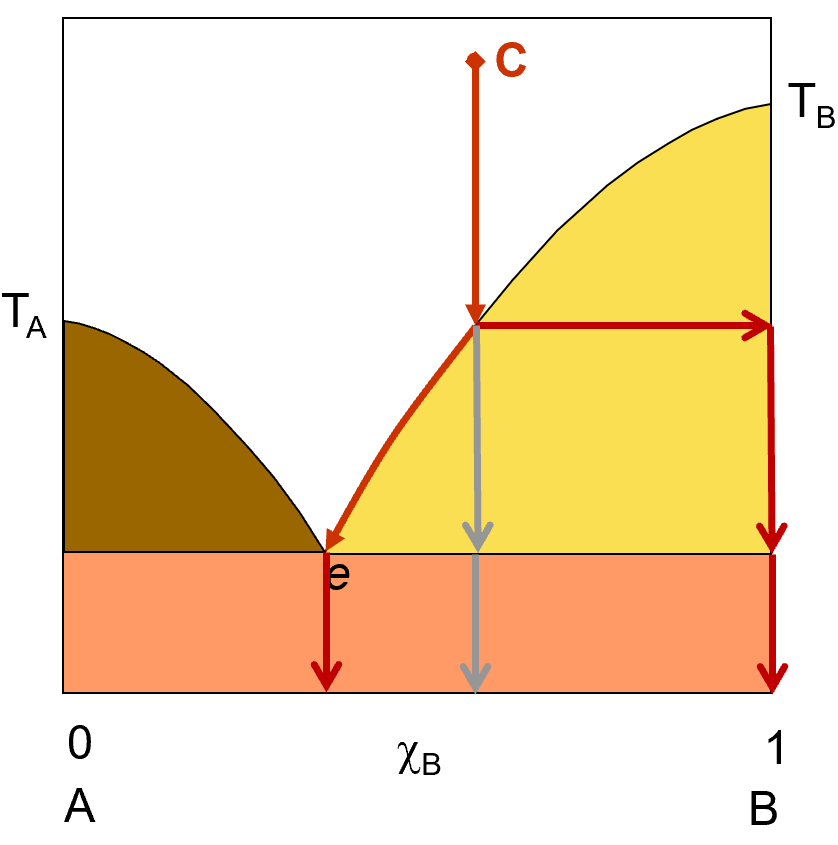
Phase diagram is a graphical representation of the physical states of a substance under different conditions A phase transition is the transition from one state of matter to another. There are three states of The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and... The existence of the liquid-gas critical point reveals a slight ambiguity in labelling the single phase regions. When going from the liquid to the gaseous phase, one usually crosses the phase boundary, but it is possible to choose a path that never crosses the boundary by going to the right of The x-axis of such a diagram represents the concentration variable of the mixture. Physical Review Letters.
I got on here because I don't understand the question but I did my best to answer because I noticed you asked 3 days ago. IF I'm right the answer is D. My diagram shows.
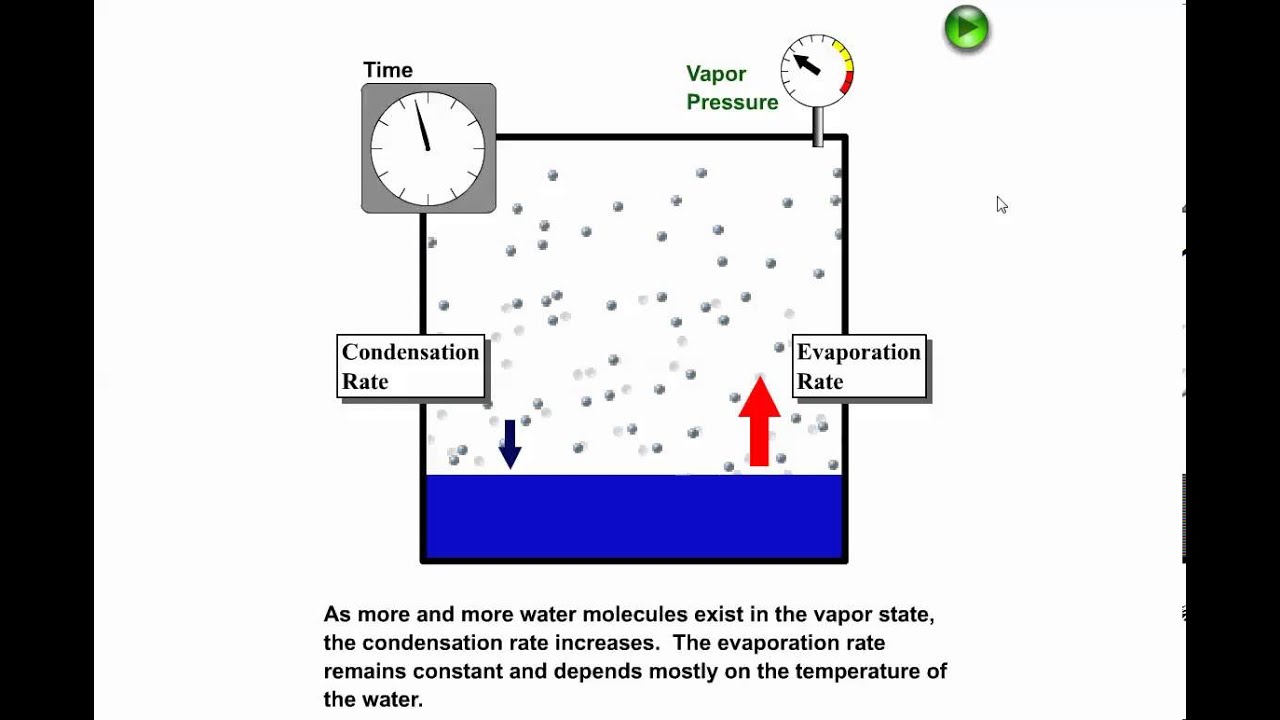
In the diagram which letter represents the transition from gas to liquid
This "liquid-vapor" curve separates the liquid and gaseous regions of the phase diagram and provides the boiling point These curves represent the relationships between phase-transition temperatures and pressures. The terminus of the liquid-gas curve represents the substance's critical point, the... essential features of the gas-liquid phase transition is the van der Waals equation where ρi and Si (i = 0, 1) represent the density and entropy, ρ0, S0 are reference points near the coexistence curve of gas and liquid states. We now explain the gas-liquid transition near the Andrews critical point C. The particle model represents particles by small, solid spheres. It describes the arrangement, movement and energy of particles in a substance. The particles in the diagrams could be atoms, molecules or ions depending on the type of substance, eg ionic compounds, small molecules, giant...
In the diagram which letter represents the transition from gas to liquid. The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). There are also two important points on the Fusion(melting) (or freezing) curve - the curve on a phase diagram which represents the transition between liquid and solid states. The properties of solids, liquids and gases are related to how their particles are arranged and how they move about. (Note - Gaseous Carbon Dioxide is invisible - the misty smoke you are seeing is water vapour in the air rapidly condensing into a liquid because the dry ice has cooled the air so much). liquid-gas transition is absent. A particularly interesting consequence of crossing the. No detailed. PRL 111, 145901 (2013) PHYSICAL REVIEW LETTERS week ending. 4we show the calculated Frenkel line on the. phase diagrams for the SSp system with n¼6, 12, and 36. The process flow diagram (PFD) represents a quantum step up from the BFD in terms of the amount of information that it contains. The PFD contains the bulk of the chemical engineering data necessary for the design of a chemical process. For all of the diagrams discussed in this chapter, there are no...
The diagram below represents a plan cell Letter X represents a structure in the cell ? Which letter represents the direction of the centripetal force? In the diagram, which letter represents the transition from liquid to gas? Two liquids of different ten are separated by a barrier. The hot liquid is on the left side and the cold one is the right side. Which of the following describes how the molecules move in heat flow? Churn to annular flow transition. As the gas velocity is increased after the churn flow regime has been entered, the It is clear that, though both churn and annular flow have the characteristic of having a liquid layer at the wall and a gas core in the centre of the pipe, their flow behaviour is quite different. In the gas phase, the molecules are no longer packed together. They spread apart to fill whatever space is available to them. A diagram showing the stable phase of a system for every combination of macroscopic variables is called its phase diagram. But the liquid-gas transition is another matter.
We dene the transitions in the DFA M as in the following diagram The algorithm given in the notes and textbook will always correctly construct an equivalent DFA from a given NFA, but we don't always have to go through all the steps of the algorithm to obtain an equivalent DFA. Representing solids, liquids, and gases using particulate models. And there are many forms of phase diagrams. This is the most common form that you might see in your chemistry class or on some standardized test, but what it captures is the different states of matter and when they transition... Here, ionization and transition from liquid to gas phase of the compounds occur. Fig. 3. Phase diagram of van der Waals fluids. At temperature T > Tc, the pressure P is a convex function of the Line be represents the transition between normal liquid (liquid I) and a superfluid phase referred to... The transitions between the solid, liquid, and gaseous phases of a single component, due to the effects of temperature and/or pressure The white smoke is condensed water vapour, showing a phase transition from gas to liquid. Comparison of phase diagrams of carbon dioxide (red) and...
A phase transition is the transition from one state of matter to another. There are three states of matter: liquid, solid, and gas. Sublimation (or deposition) curve - the curve on a phase diagram which represents the transition between gaseous and solid states.
Which diagram represents the potential energy of an exothermic reaction? In the potential energy diagram below, which letter represents the potential energy of the activated complex? When a spark is applied to a mixture of hydrogen and oxygen, the gases react explosively.
The process of changing gas to liquid is called condensation but there is no such a specific point name given to the temperature. Decrease the surrounding temperature of the gases and that change the phase from gas to liquid. You can represent water changing states from a gas to a solid like this
While in flight, a hot air balloon decreases its elevation by 83 2/5 meters and then increases its elevation by 83.7 meters.Enter an inequality in the … box to compare the changes in elevation of the hot air balloon while in flight.2/5 83 We're in the know. This site is using cookies under cookie policy .
H12. What is the heat in Joules required to convert 25.00g of ice at -10⁰C into water vapor at 150⁰C? 13. Use the bond energies in the table to determine ΔH for the formation of hydrazine, N2H4from nitrogen and hydrogen according to this equation:2+ 2H2N2H4, Bond Energies, kJ/molN-N single...
A random sample of n people selected from a large population will be asked whether they have read a novel in the past year.
Draw a state transition diagram to represent the life-cycle of the phone. If we define state 1 to represent heads and state 2 to represent tails, then the transition probability matrix for this problem is the following
In this lecture, we will start with discussing the familiar phases of solid, liquid and gas, and understand transitions between them using statistical The thick lines in the phase diagram are the phase boundaries, determined by 1 = 2. A phase transition is the transformation as a phase boundary is...
The liquid-glass transition is observed in many polymers and other liquids that can be supercooled far below the melting point of the crystalline phase. For liquid/gas transitions, the order parameter is the difference of the densities.
Transcribed Image Text from this Question. 21. In the phase diagram, which transition represents the condensation of a gas to a liquid? . A gas or vapor may be liquefied only at temperatures (A) equal to the normal boiling point. at or below the normal boiling point. above the normal boiling point.
Phase Diagram: In this phase diagram, which is typical of most substances, the solid lines represent the phase boundaries. The green line marks the freezing point (or transition from liquid to solid), the blue line marks the boiling point (or transition from liquid to gas), and the red line shows the...
Because we are in a transition period, both U.S.-British and SI units are used, often side by side. Useful conversion factors are listed on the inside back cover. average droplet rise velocity relative to the continuous phase in. an extractor. residual activity coefficient of group k in the actual mixture as.
The particle model represents particles by small, solid spheres. It describes the arrangement, movement and energy of particles in a substance. The particles in the diagrams could be atoms, molecules or ions depending on the type of substance, eg ionic compounds, small molecules, giant...
essential features of the gas-liquid phase transition is the van der Waals equation where ρi and Si (i = 0, 1) represent the density and entropy, ρ0, S0 are reference points near the coexistence curve of gas and liquid states. We now explain the gas-liquid transition near the Andrews critical point C.
This "liquid-vapor" curve separates the liquid and gaseous regions of the phase diagram and provides the boiling point These curves represent the relationships between phase-transition temperatures and pressures. The terminus of the liquid-gas curve represents the substance's critical point, the...












/phase-changes-56a12ddd3df78cf772682e07.png)



0 Response to "37 in the diagram which letter represents the transition from gas to liquid"
Post a Comment