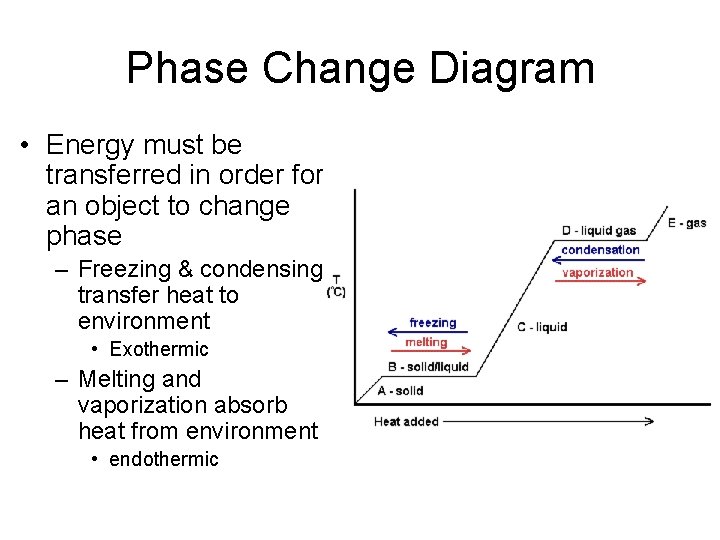
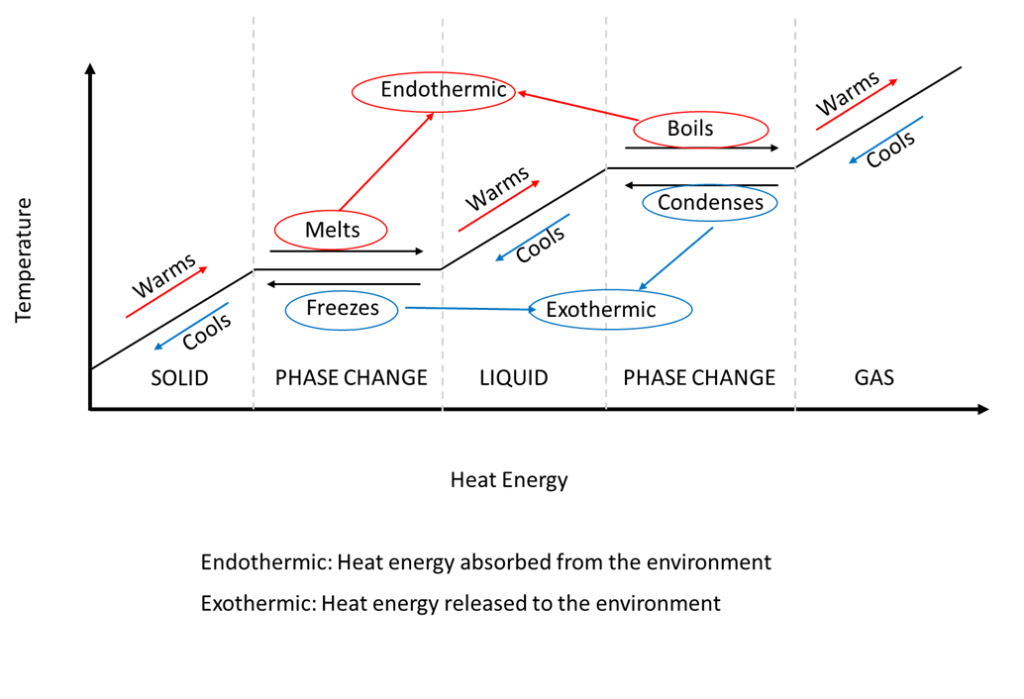
39 phase change diagram endothermic exothermic
Endothermic and Exothermic ... During a phase change, the heat added (PE increases) or ... What sections of the graph have constant kinetic energy?7 pages A phase change is a physical process in which a substance goes from one phase to another. Oct 18, 2004 · Inside the Alinea Food Lab. 75 1. . 4 – I can record observations and experimental data neatly and accurately Sign in to your DocuSign account to electronically sign documents, request signatures, check document status, send reminders ...
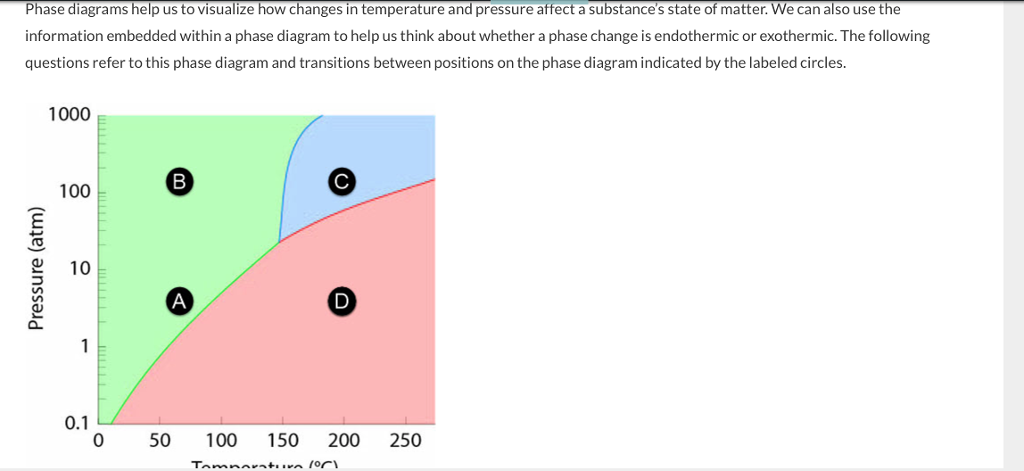
Aug 01, 2014 · Phase change materials (PCMs) used for the storage of thermal energy as sensible and latent heat are an important class of modern materials which substantially contribute to the efficient use and conservation of waste heat and solar energy. The storage of latent heat provides a greater density of energy storage with a smaller temperature ...
Phase change diagram endothermic exothermic
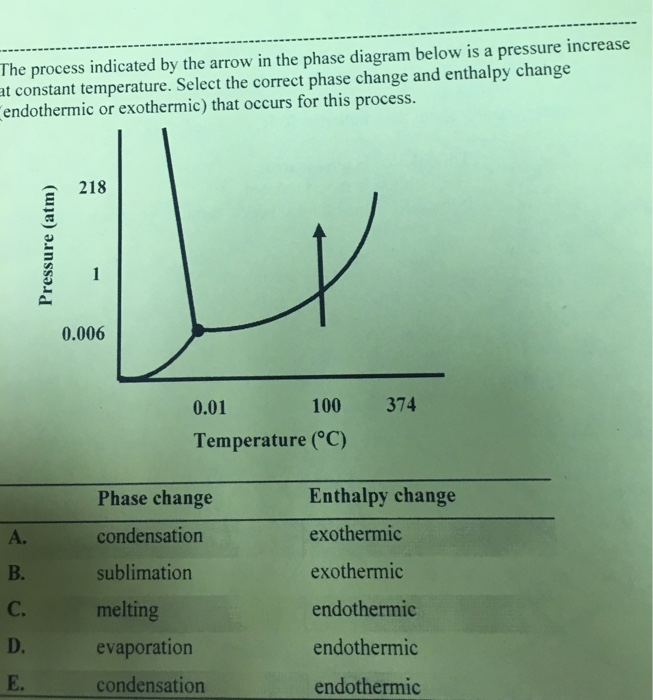
Base your answers on the information and diagram below, which represent the changes in potential energy that occur during the given reaction. Given the reaction: A + B --> C. a) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer. Mar 19, 2003 — When attempting to understand the phase changes it is important to remember what is occurring in endo and exothermic reactions. Endothermic - ... c. 1200, "to alter, make different, change" (transitive); early 13c. as "to substitute one for another;" mid-13c. as "to make (something) other than what it was, cause to turn or pass from one state to another;" from late 13c. as "to become different, be altered" (intransitive), from Old French changier "to change, alter; exchange, switch," from Late Latin cambiare "to barter, exchange," extended form of Latin cambire "to exchange, barter," a word of Celtic origin, from PIE root *kemb- "to bend, crook" (with a sense evolution perhaps from "to turn" to "to change," to "to barter"); cognate with Old Irish camm "crooked, curved;" Middle Irish cimb "tribute," cimbid "prisoner;" see cant (n.2). From c. 1300 as "undergo alteration, become different." In part an abbreviation of exchange. From late 14c. especially "to give an equivalent for in smaller parts of the same kind" (money). Meaning "to take off clothes and put on other ones" is from late 15c. Related: Changed;changing. To change (one's) mind is from 1590s.
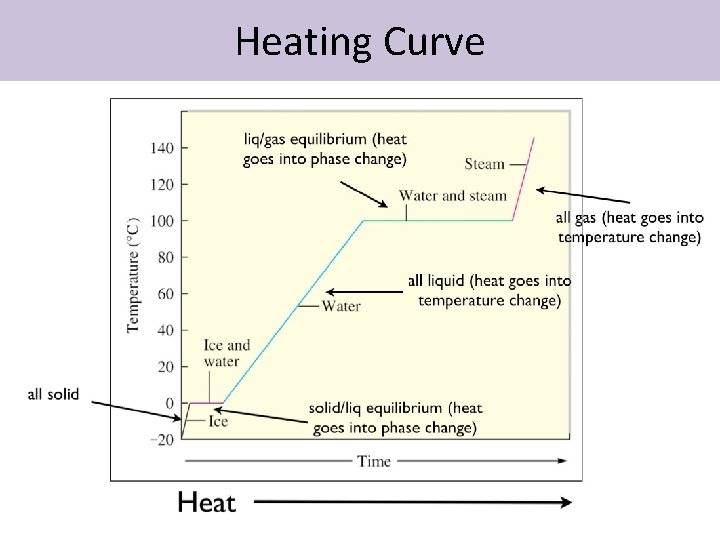
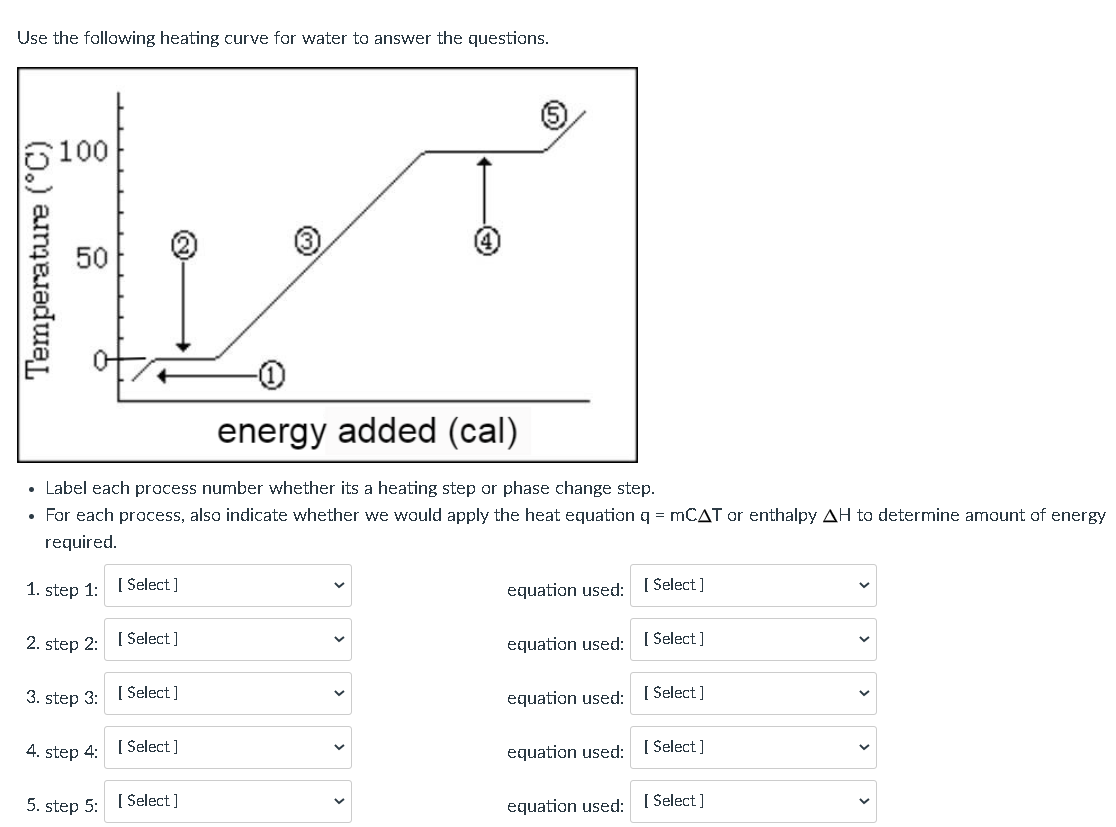
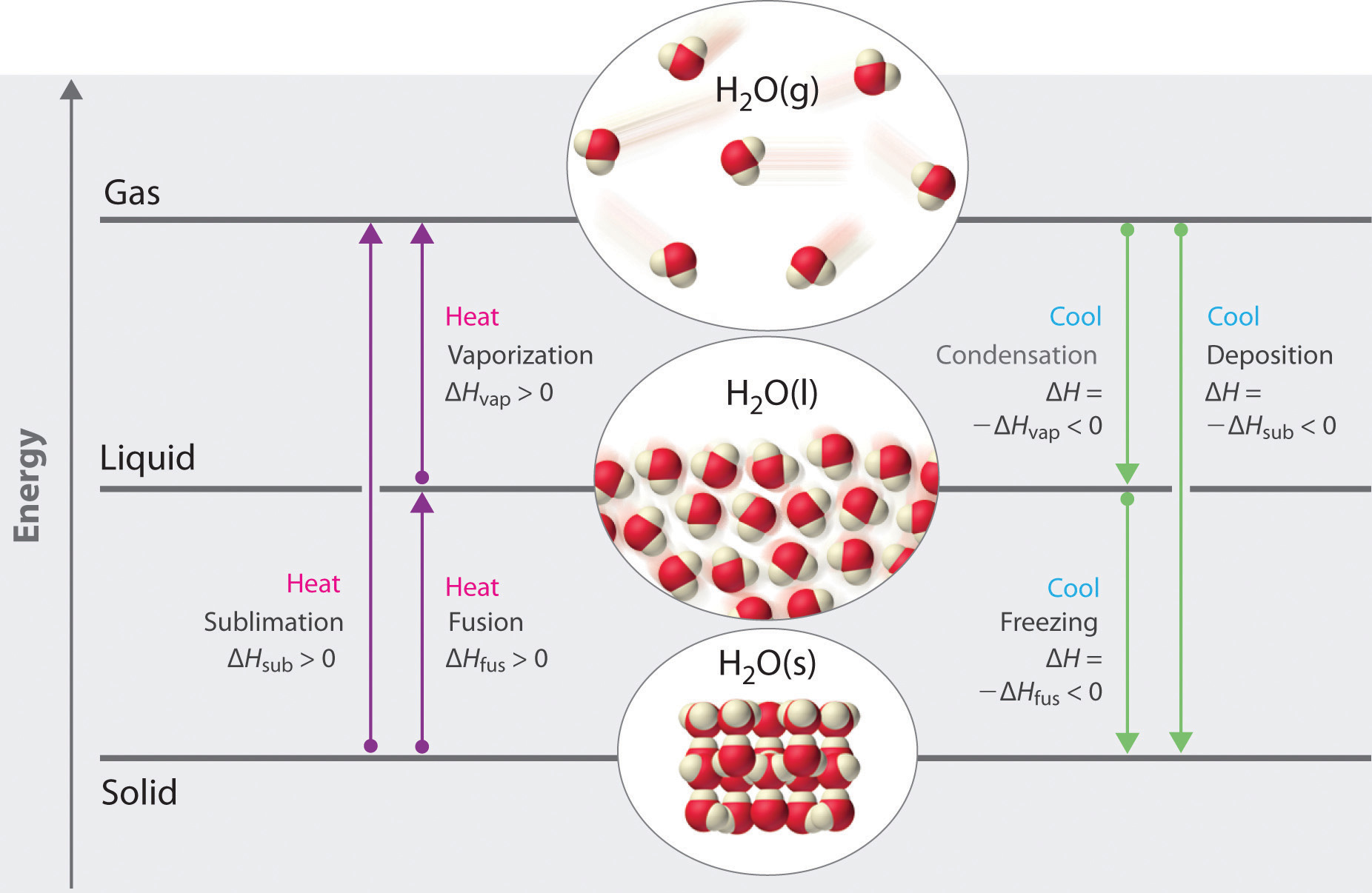
Phase change diagram endothermic exothermic. "to synchronize, adjust the phase of so as to synchronize," 1895, from phase (n.) in the physics sense of "particular stage or point in a recurring sequence of movement or changes" (1861). Earlier as a bad spelling of faze. Meaning "to carry out gradually" is from 1949, hence phase in "introduce gradually" (1954), phase out "take out gradually in planned stages" (1954). Related: Phased; phasing. 1705, "phase of the moon, particular recurrent appearance presented by the moon (or Mercury or Venus) at a particular time," back-formed as a singular from Modern Latin phases, plural of phasis, from Greek phasis "appearance" (of a star), "phase" (of the moon), from stem of phainein "to show, to make appear" (from PIE root *bha- (1) "to shine"). Latin singular phasis was used in English from 1660 for each of the aspects of the moon. General (non-lunar) sense of "aspect, appearance, stage of development at a particular time" is attested by 1841. Meaning "temporary difficult period" (especially in reference to adolescents) is attested from 1913. Phase Change Diagram. The graph was drawn from data collected as 1 mole of a substance was heated at a constant rate. Use the graph to answer the following questions. ... Is vaporization endothermic or exothermic? Explain. Describe the process of sublimation and give an example. Is sublimation endothermic or exothermic? Explain. Recall that endothermic processes have a positive enthalpy change, and exothermic processes have a negative enthalpy change. Thermodynamic (Macroscopic) View In addition to the microscopic view presented above, we can describe phase transitions in terms of macroscopic, thermodynamic properties.
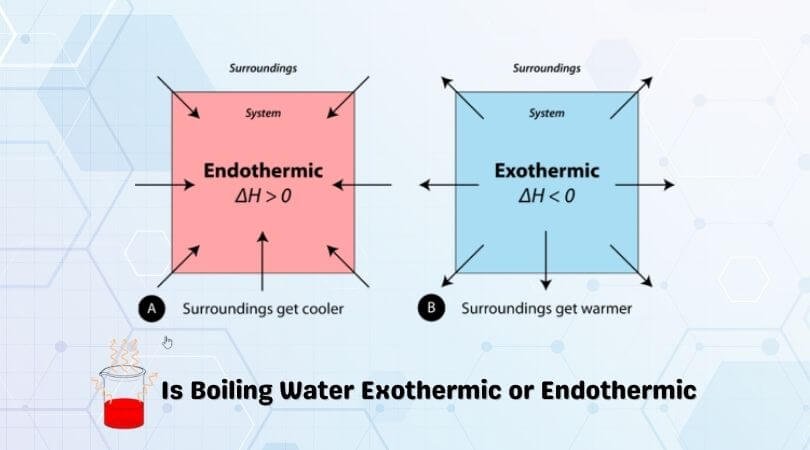
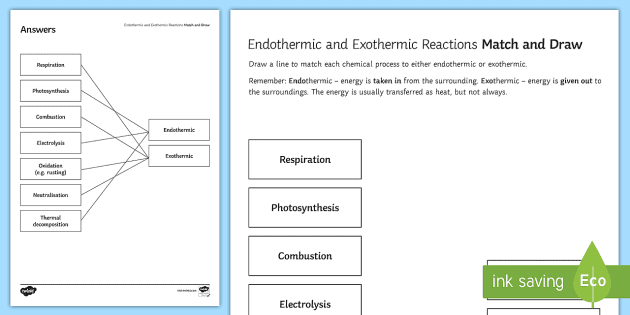
A. the reaction is exothermic B. the reaction is endothermic C. the reactants lost internal energy D. the change in enthalpy is positive. A, C. ... Consider the phase change H2O(l) H2O(g). This equation describes the phase change from liquid water to steam. ... From the diagram, when the temperature of reactants are raised from 20°C to 30°C: ... during phase changes in the diagram below. For more information on this Reading Strategy, see the Reading and Study Skills in the Skills and Reference Handbook at the end of your textbook. Endothermic Exothermic Characteristics of Phase Changes (pages … Nov 22, 2021 · The categorization of a reaction as endo- or exothermic dependsAmmonium nitrate, $\ce{NH4NO3}$, is a good example of a salt which dissolves in water in an endothermic reaction: if you dissolve some $\ce{NH4NO3}$ in water, the drop in the temperature will be noticeable Which of the following best describes the potential energy diagram of an ... 1869, originally in chemistry, "causing, relating to, or requiring the absorption of heat," from French endothermique (1868, Berthelot); see endo- + thermal. By 1947 in biology, "dependent on or capable of the internal generation of heat; warm-blooded."
Feb 14, 2020 — Here is how you would classify the phase changes as endothermic or exothermic: melting, evaporation and sublimation are endothermic ... 1918 (Venn's diagram is from 1904), named for English logician John Venn (1834-1923) of Cambridge, who explained them in the book "Symbolic Logic" (1881). c. 1200, "act or fact of changing," from Anglo-French chaunge, Old French change "exchange, recompense, reciprocation," from changier "to alter; exchange; to switch" (see change (v.)). Related: changes. Meaning "a different situation, variety, novelty" is from 1680s (as in for a change, 1690s). Meaning "something substituted for something else" is from 1590s. Meaning "place where merchants meet to do business" is from c. 1400. Meaning "the passing from life to death" is biblical (161os). The financial sense of "balance of money returned after deducting the price of a purchase from the sum paid" is first recorded 1620s; hence to make change (by 1865). Bell-ringing sense is from 1610s, "any sequence other than the diatonic." Hence the figurative phrase ring changes "repeat in every possible order" (1610s). Figurative phrase change of heart is from 1828. In reference to women, change of life "final cessation of menstruation" is recorded from 1834. 1610s, "an illustrative figure giving only the outlines or general scheme of the object;" 1640s in geometry, "a drawing for the purpose of demonstrating the properties of a figure;" from French diagramme, from Latin diagramma "a scale, a musical scale," from Greek diagramma "geometric figure, that which is marked out by lines," from diagraphein "mark out by lines, delineate," from dia "across, through" (see dia-) + graphein "write, mark, draw" (see -graphy). Related: Diagrammatic; diagrammatically. The verb, "to draw or put in the form of a diagram," is by 1822, from the noun. Related: Diagrammed; diagramming.
"gradual, planned removal or elimination," 1958, from the verbal phrase (1954; see phase (v.)).
Other Endothermic Processes. The melting of ice to form water. Evaporation of liquid water, forming water vapour. Sublimation of solid CO 2; The baking of bread. Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below.
I'm tutoring someone who is struggling with topics from content category 5E. I already recommended Leah4Sci and they are already using Jack Westin. What other resources did you like for learning these topics besides simply reading a review book? ​ ***Content Category 5E: Principles of chemical thermodynamics and kinetics*** **Enzymes (BC, BIO)** * [Classification by reaction type](https://jackwestin.com/resources/mcat-content/enzymes/classification-by-reaction-type) * [Mechanism]...
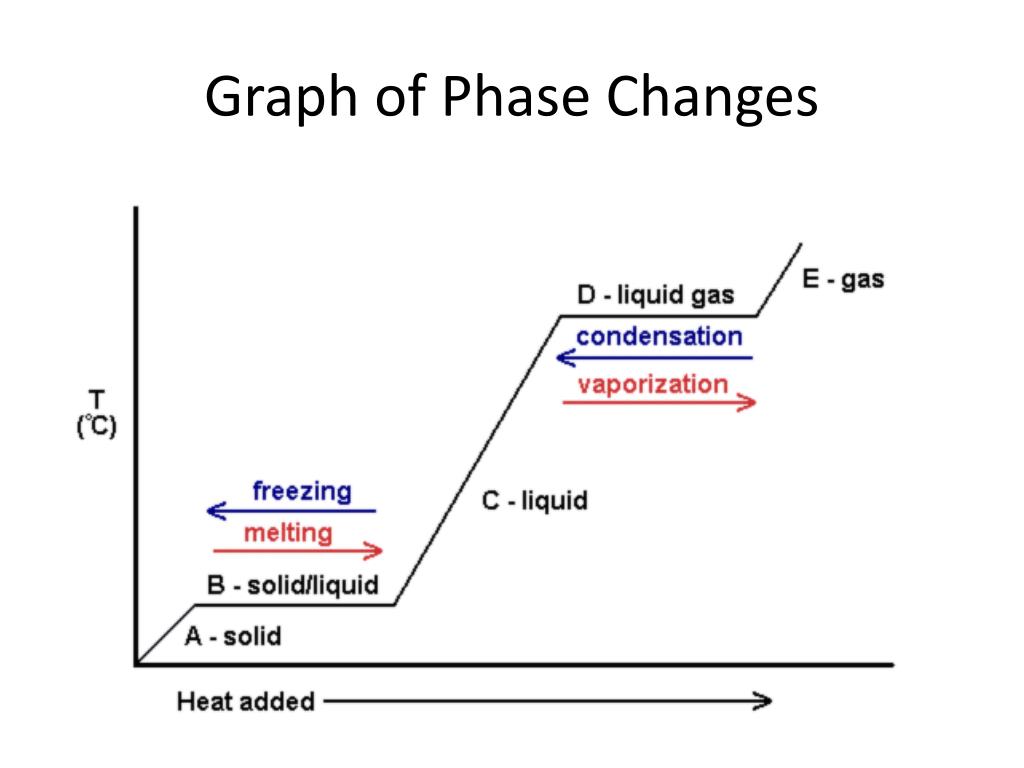
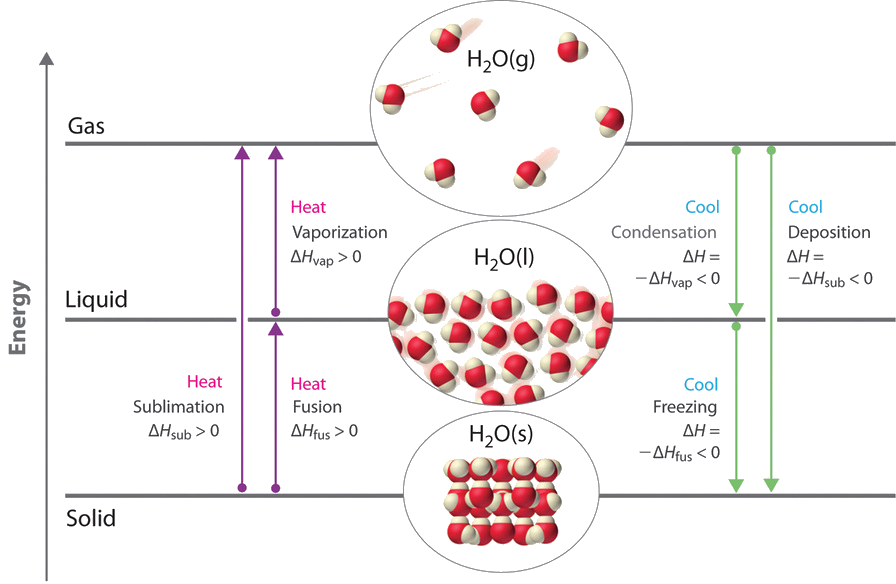
Nov 6, 2021 — Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes.Energy Changes That... · Temperature Curves · Heating Curves
Substances can change phase—often because of a temperature change. ... Because the substance is melting, the process is endothermic, so the energy change ...
Recall that endothermic processes have a positive enthalpy change, and exothermic processes have a negative enthalpy change. As with other chemical reactions, because enthalpy is a state function, ΔH for phase transitions can be added or subtracted according to Hess's law.
In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, ...
also shortchange, "to cheat by giving too little change to," 1903, from adjectival expression short-change (with man, trick, etc.), 1901, from short (adj.) + change (n.).
1874, in chemistry, "relating to a liberation of heat," modeled on French exothermique (1869, Berthelot); see exo- + thermal.
c. 1200, "to alter, make different, change" (transitive); early 13c. as "to substitute one for another;" mid-13c. as "to make (something) other than what it was, cause to turn or pass from one state to another;" from late 13c. as "to become different, be altered" (intransitive), from Old French changier "to change, alter; exchange, switch," from Late Latin cambiare "to barter, exchange," extended form of Latin cambire "to exchange, barter," a word of Celtic origin, from PIE root *kemb- "to bend, crook" (with a sense evolution perhaps from "to turn" to "to change," to "to barter"); cognate with Old Irish camm "crooked, curved;" Middle Irish cimb "tribute," cimbid "prisoner;" see cant (n.2). From c. 1300 as "undergo alteration, become different." In part an abbreviation of exchange. From late 14c. especially "to give an equivalent for in smaller parts of the same kind" (money). Meaning "to take off clothes and put on other ones" is from late 15c. Related: Changed;changing. To change (one's) mind is from 1590s.
Mar 19, 2003 — When attempting to understand the phase changes it is important to remember what is occurring in endo and exothermic reactions. Endothermic - ...
Base your answers on the information and diagram below, which represent the changes in potential energy that occur during the given reaction. Given the reaction: A + B --> C. a) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer.



















0 Response to "39 phase change diagram endothermic exothermic"
Post a Comment