40 molecular orbital diagram for h2
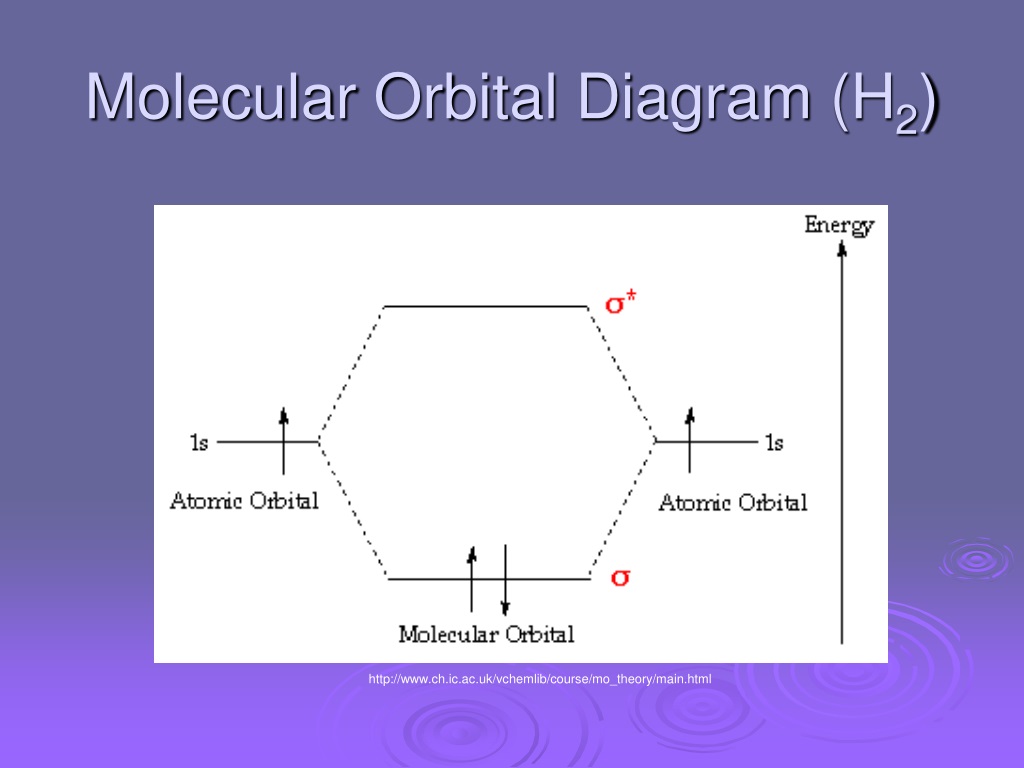
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. The bonding molecular orbital concentrates electrons in the region directly between the two nuclei. Placing an electron in this orbital therefore stabilizes the H2 molecule. This diagram suggests that the energy of an H2 molecule is lower than that of a pair of isolated atoms.
Molecular orbital mo theory of the h2 molecule. Discussed in this video are. H2 Ion Molecular Orbital Diagram Wiring Diagra...
Molecular orbital diagram for h2
C would this ion exist. In fact they do. Energy Level Diagram For Molecular Orbitals Chemical Bonding And Constr... H2. Lewis Structure: Molecular Orbital Energy Diagram. The orbital correlation diagram for diboron, however, is not generally applicable for all homonuclear diatomic molecules. It turns out that only when the bond lengths are relatively short (as in B2, C2, and N2) can the two p-orbitals on the... : Molecular Orbitals for the H 2 Molecule. (a) This diagram shows the formation of a bonding σ 1 s molecular orbital for H 2 as the sum of the wave functions (Ψ) of two H 1 s atomic orbitals. (b) This plot of the square of the wave function (Ψ 2...
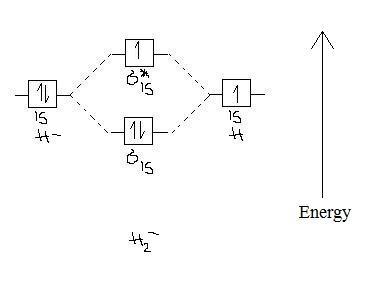
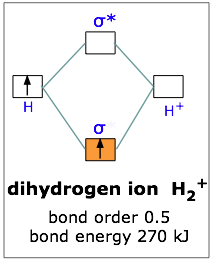
Molecular orbital diagram for h2. The molecular orbital electronic configuration of Homonuclear Diatomic Molecule H2 is: Therefore, there is a covalent bond between the two hydrogen atoms. No. 3 Molecular OrbitalEnergy Diagram for He2 molecule (hypothetical). There is no net bonding as it has zero bond order and therefore He2... About molecular orbitals. The simplest molecule: H2+. Bonding and antibonding orbitals. Simple molecular orbital diagrams. The geometric mean of the H2 and Li2 bond energies is 213 kJ/mole, so it appears that the lithium hydride molecule is 30 kJ/mole more stable than it "is supposed" to be. Science. Chemistry Q&A Library Construct the molecular orbital diagram for H2. Identify the bond order. Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me...
Figure 8.35 The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower energy than the separated atoms. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule. In molecular orbital theory, bond order is also defined as the difference, divided by two, between the number of bonding and antibonding electrons; this This MO diagram depicts the molecule H2, with the contributing AOs on the outside sandwiching the MO. The bonding level (lower level) is completely... Molecular Orbital Treatment Without going into the group theory considerations of how to set up symmetry adapted atomic orbitals on the metals and the ligands. How would one go about trying to build a molecular orbital diagram for a coordination. Molecular Orbital Theory. I'm having a lot of trouble with this stuff. I don't really know how to start these questions (such as how to draw So here, I basically ask, how do I draw a correlation diagram? Like, how do I know how many electrons to put in the bonding atomic orbital and antibonding atomic orbital?
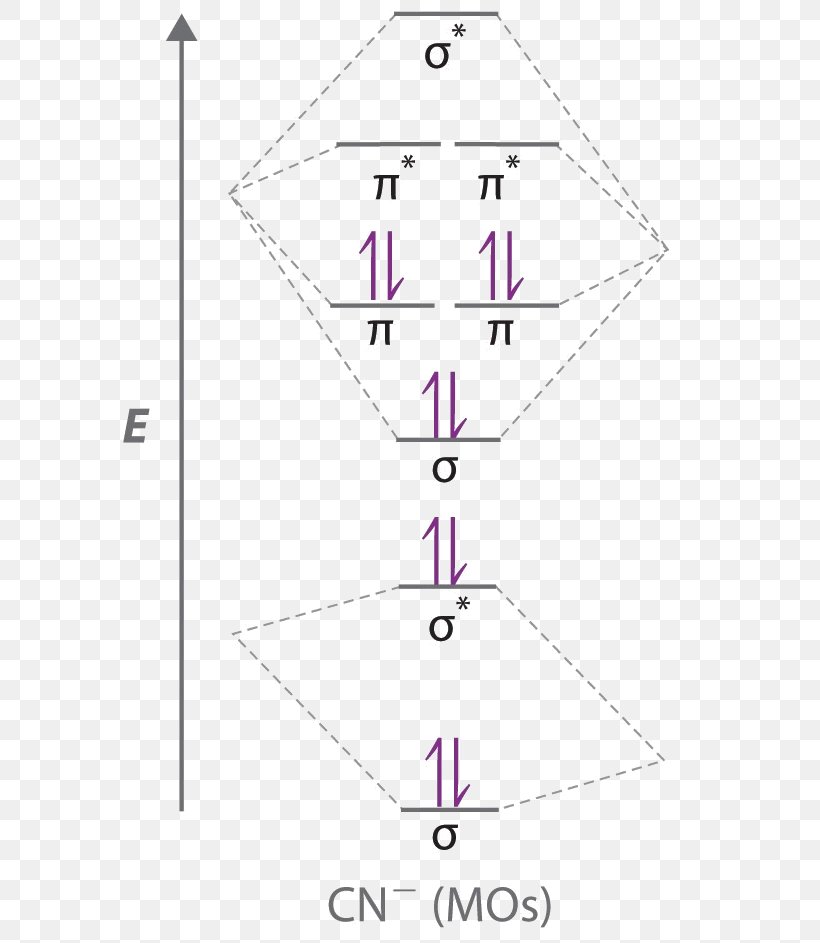
• Bonding - Review VSEPR and Hybridisation - Linear combination of molecular orbitals (LCAO), bonding / antibonding - Labelling of molecular orbitals (MOs) (σ, π and g, u) - Homonuclear diatomic MO diagrams - mixing of different AO's - More complex molecules (CO, H2O ….) In fact they do. The lewis structure for h2 is h h predicting a single bond between each hydrogen atom with two electrons in the bond. Click thin the blue boxes to add electrons. Bonding mos antibonding mos and bond order. Mo Diagram Of B 2 H 6 Example... The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in Electrons in the bonding orbital strengthen the bond; electrons in the antibonding orbital weaken the bond, H2 has just enough electrons (two) to fill...
Molecular orbital diagram for hydrogen gas h2. A bonding mo and an anti bonding mo. 1 12 and 12 c. When the 1s wave functions A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and...
Figure 9. The molecular orbital energy diagram predicts that H2 will be a stable molecule with lower energy than the separated atoms. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule.
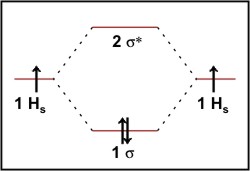
Figure 13: A molecular orbital energy-level diagram showing the relative energies of the atomic orbitals of atoms A and B (1sA and 1sB) and the bonding (1σ) and antibonding (2σ) molecular orbitals they form.
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
Molecular orbital mo theory of the h2 molecule. Mo diagrams can explain why some molecules exist and others do not.
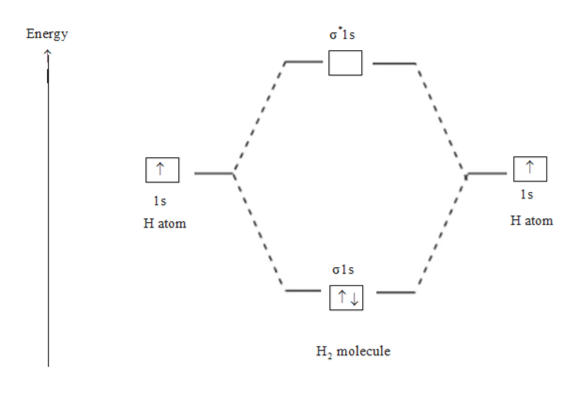
Figure 9-2 Molecular orbital (MO) diagram for the combination of the 1s atomic orbitals on two identical atoms (at the left) to form two MOs. Because the two electrons in an H2 molecule are in a bonding orbital, the bond order is one. We conclude that the H2 molecule would be stable, and we...
Because of their simplicity they have been extensively studied. Bonding order is 1 and it is diamagnetic. Mo Theory...
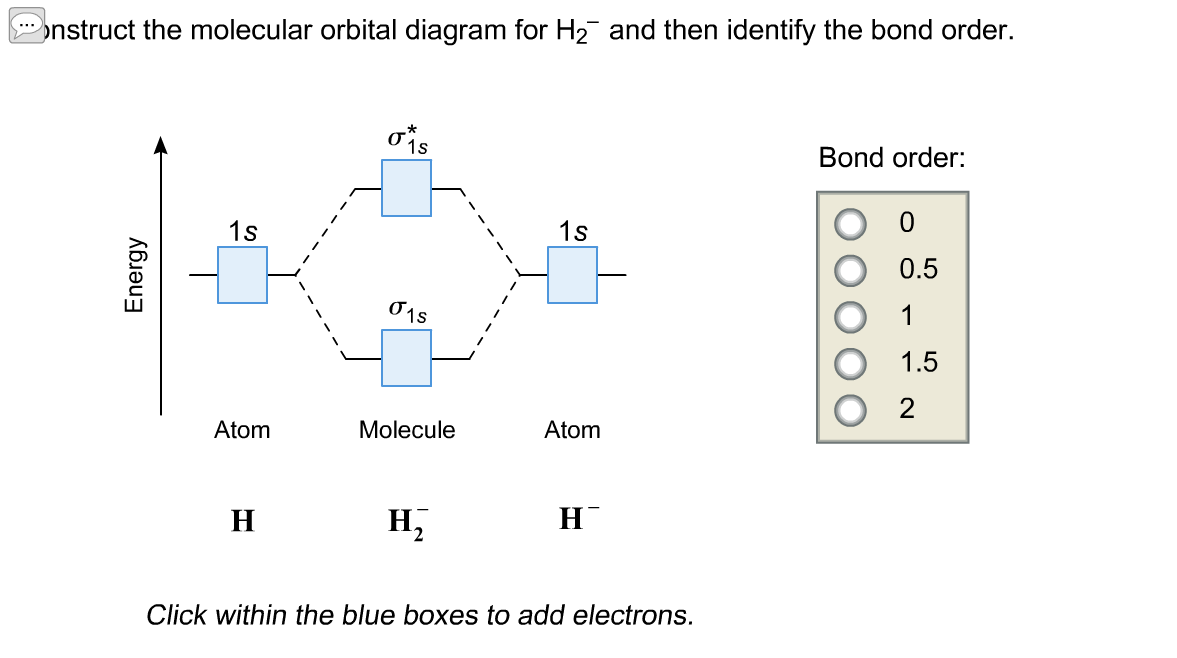
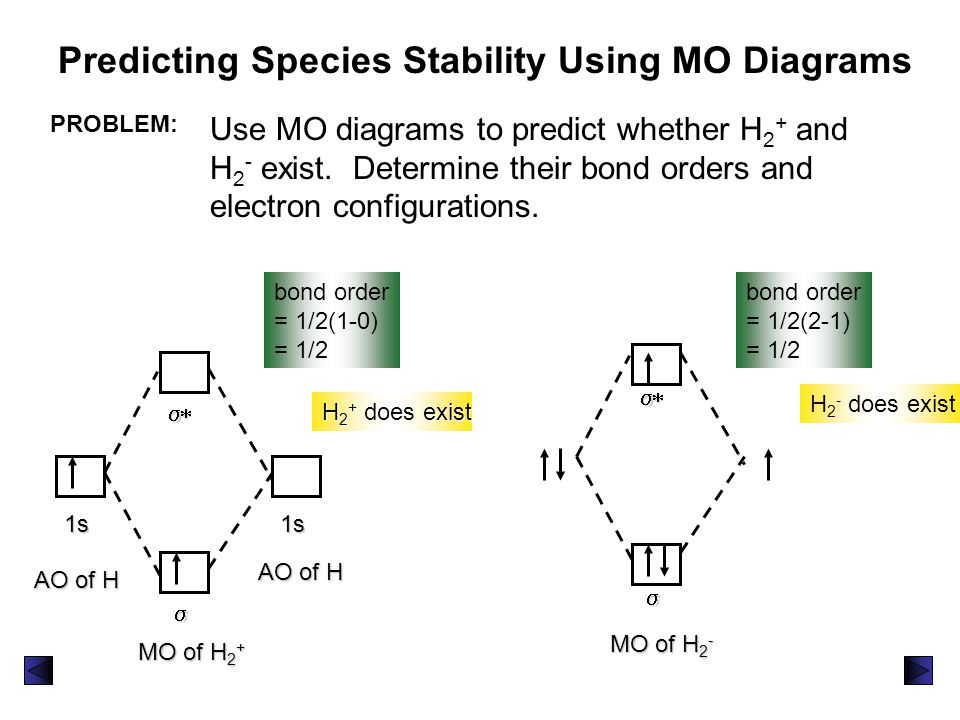
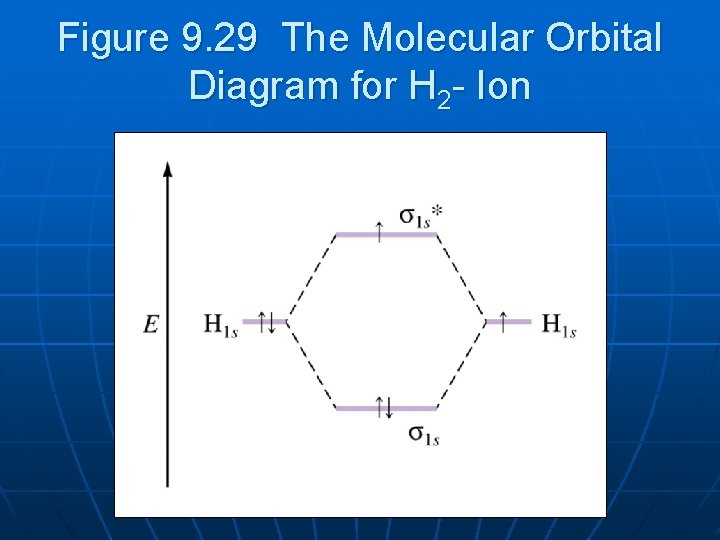
Transcribed image text : Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: 0 0.5 1 1.5 2 Click within the blue boxes to add electrons.
Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Molecular orbital diagram for hydrogen gas h2. Molecular orbital diagrams of diatomic molecules. Solved In Class We Looked...
Each orbital accommodates two electrons and the two electrons in ceh h fill the s 1s molecular orbital mo. This tool is very.
: Molecular Orbitals for the H 2 Molecule. (a) This diagram shows the formation of a bonding σ 1 s molecular orbital for H 2 as the sum of the wave functions (Ψ) of two H 1 s atomic orbitals. (b) This plot of the square of the wave function (Ψ 2...
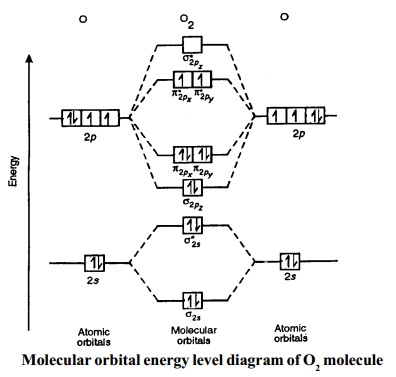
H2. Lewis Structure: Molecular Orbital Energy Diagram. The orbital correlation diagram for diboron, however, is not generally applicable for all homonuclear diatomic molecules. It turns out that only when the bond lengths are relatively short (as in B2, C2, and N2) can the two p-orbitals on the...
C would this ion exist. In fact they do. Energy Level Diagram For Molecular Orbitals Chemical Bonding And Constr...
























0 Response to "40 molecular orbital diagram for h2"
Post a Comment