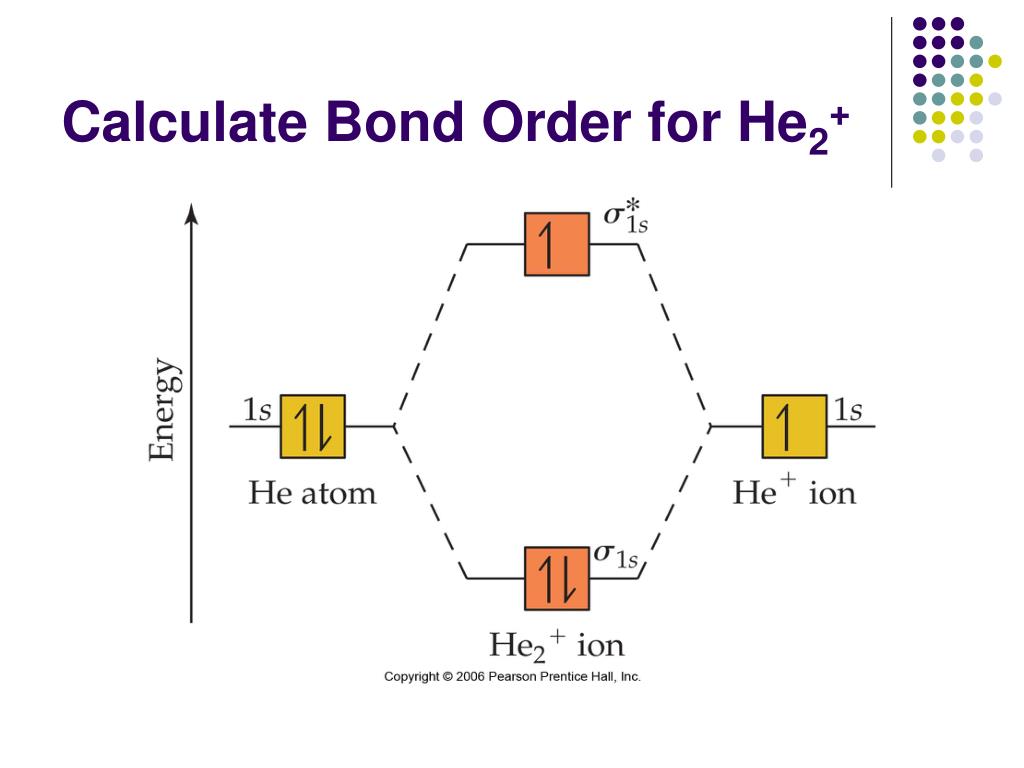
39 molecular orbital diagram for he2+
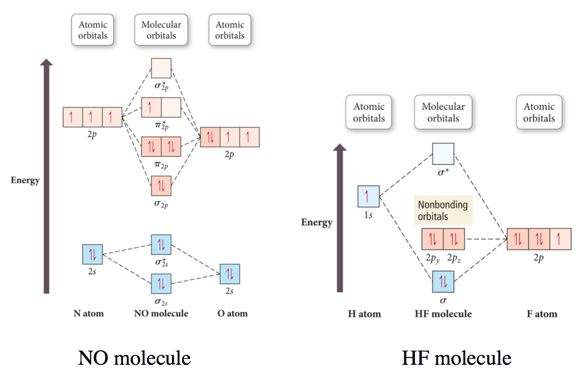
2n=6 Mitosis Diagram - schematron.org Mar 16, 2019 · Construct The Molecular Orbital Diagram For He2 And Then Identify The Bond Order. Astak Cm 918t Wiring Diagram; Fj Cruiser Serpentine Belt Diagram; Doosan D80s Forklift Wiring Diagram; Kenwood Kdc 2011s Wiring Diagram; Ezgo Txt 36 Volt Shift Lever Wiring Diagram; Suzuki Eiger 400 Wiring Diagram And Parts; Arduino Uno Dm542t Wiring Diagram chemical bonding - Molecular orbitals of H2 and He2 ... The molecular orbital energy-level diagram shown in Figure 13 also applies (with changes of detail in the energies of the molecular orbitals) to the hypothetical species He 2. However, this species has four valence electrons, and its configuration would be 1σ 2 2σ 2.
Mo Diagram He2 The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding.
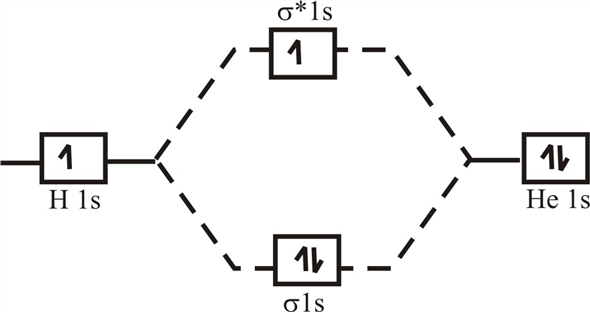
Molecular orbital diagram for he2+
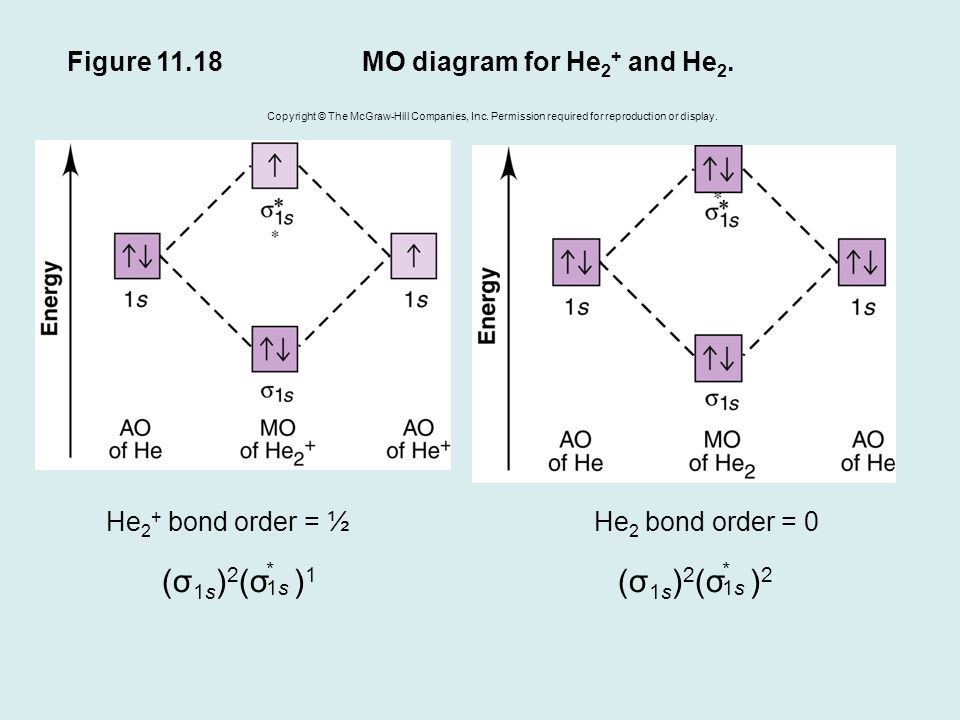
Molecular Orbital Diagram For He2 A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 . Molecular Orbital Theory The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons - Number of Antibonding Electrons) = 1/2 x (2-2)=0. Molecular Orbital Diagram For He2+ - schematron.org He2+ MO diagram. Eg: Li + H; Li has 1s + 2s, while H has 1s. This mix to form a sigma orbital from H1s+Li2s, a sigma* orbital and H1s-Li2s. The bond order of a simple molecule can be determined by looking at the number of electrons in bonding and antibonding molecular orbitals. Like electrons in.
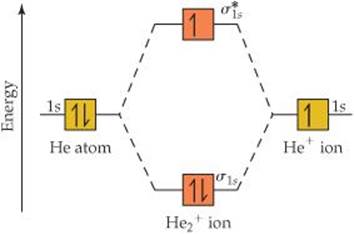
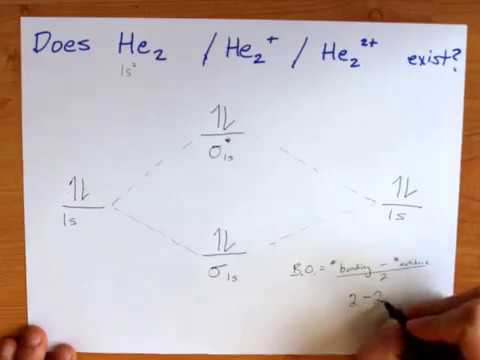
Molecular orbital diagram for he2+. CBSE Class 11 Chemistry Notes Download in PDF | Toppers CBSE ... CBSE class 11 Chemistry Notes download in PDF. CBSE class 11 Chemistry notes prepared with colored diagrams, easy language. Achieve 100% preparations. 40 molecular orbital diagram for he2+ - Diagram For You Molecular orbital diagram for he2+. What Is The Ground State Electron Configuration And The ... So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). Why is He2 not a stable molecule? - CHEMISTRY COMMUNITY To answer the question, you must construct a molecular orbital (MO) diagram for the hypothetical He 2 molecule. The σ 1s bonding and antibonding orbitals will be full. Calculating the bond order results in 0. In other words, no bond can be sustained between two He atoms according to MO theory. Top. He2 2+ Molecular Orbital Diagram - schematron.org (a) The H 2 + ion. A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.
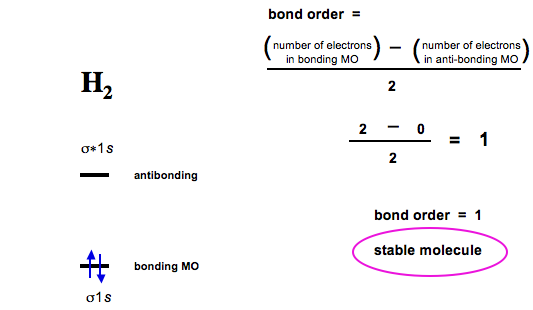
Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration. 7.7 Molecular Orbital Theory - Chemistry Fundamentals A molecular orbital can hold two electrons, so both electrons in the H 2 molecule are in the [latex]\sigma[/latex] 1s bonding orbital; the electron configuration is [latex]{\left({\sigma}_{1s}\right)}^{2}.[/latex] We represent this configuration by a molecular orbital energy diagram (Figure 7.7.10) in which a single upward arrow indicates one ... Why does He2 not exist? - Quora Molecular orbital diagram for He₂ can be given as. σ1s² σ*1s² Thus it's bond order is B.O. = 1/2 (2-2) = 0 The zero bond order indicates that the molecule does not exist. Calculations have shown that the antibonding effect of antibonding MO is stronger than the bonding effect of a bonding MO. Do He2, He2(+), He2(2+) exist, stable? (Molecular Orbital ... In He2 (dihelium), the two 1s atomic orbitals overlap to create two molecular orbitals: sigma(1s) and sigma(1s)*. You fill these molecular orbitals with the...
Molecular orbital diagram of h2 - Soetrust Construct the molecular orbital diagram for he2; Use the molecular orbital diagram shown to determine which… using the molecular orbital theory, describe the bonding in… Write orbital diagram for Au+? Decide if N2 and N2+ are paramagnetic or diamagnetic. Which… What is the bond order of C2 2- ? Molecular Orbital Diagram For He2 2+ - Wiring Diagrams Please note the diagram is for He2+ but the He-H is very similar answered Mar 21 '13 at A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−. He2 2+ Molecular Orbital Diagram - Wiring Diagrams He2 2+ Molecular Orbital Diagram The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H2 molecule is shown in Figure Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. Why He2 molecule does not exist? Explain by MOT. Solution Verified by Toppr Electronic configuration of He is 1s 2. Molecular Orbital Diagram for He 2 is (Refer to Image) Bond order= 2(No. of electrons in bonding molecular orbital)- (No. of electrons in anti-bonding Molecular orbital) = 22−2 =0 ∴He 2 bond order is 0. There is no bond existing between atoms of He 2 . So He 2 does not exist.
What is the MOED of He2 molecule class 11 chemistry CBSE Hint: As we know that molecular orbital theory assumes that in molecules the atomic orbitals lose their identity and the electrons in molecules are present in new orbitals called molecular orbitals. Molecular orbitals energy diagrams show the relative energies of molecular orbitals. Complete step by step answer: The molecular orbital theory assumes that the atomic orbitals in molecules lose ...
Molecular orbital correlation diagrams for He2, He2+, N2 ... Ionization potential curves plotted on the diagrams for the neutral systems are nearly parallel to the corresponding orbital energy curves. This indicates that Koopmans' theorem is consistent at all distances and implies that correlation diagrams for neutral molecules should be reasonable approximations to the ionization potential curves.

Example #3: Fill in the molecular orbital diagram for when two carbon atoms combine their valence electrons.
Module Two Chem 101 Problems Flashcards - Quizlet The molecular orbital energy diagram for N₂ is shown below. Based on this diagram, is the molecule paramagnetic or diamagnetic? ... C. He2 Draw the MO diagram for ...
3 Ways to Calculate Bond Order in Chemistry - wikiHow Jan 18, 2022 · In molecular orbital theory, bond order is also defined as half of the difference between the number of bonding and antibonding electrons. For a straightforward answer: use this formula: Bond order = [(Number of electrons in bonding molecules) - (Number of electrons in antibonding molecules)]/2 .
He2 - CHEMISTRY COMMUNITY There is a note in the course reader that says He2 is unstable, why is this? When I completed the molecular orbital diagram all the orbitals are filled and it is diamagnetic. Top. Hannah_1C Posts: 5 Joined: Fri Sep 20, 2013 10:00 am. Re: He2. Post by Hannah_1C » Wed Jul 27, 2016 8:16 am .
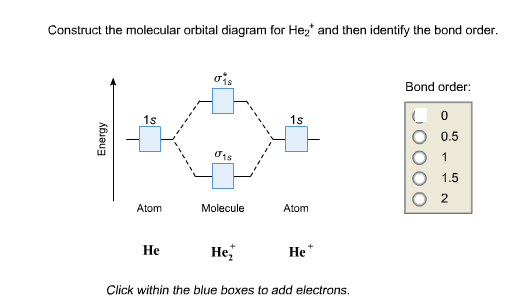
Solved Construct the molecular orbital diagram for He2 and ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 80% (83 ratings) Transcribed image text: Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxes to add electrons.
Molecular orbital diarams for He2 and He2+ - YouTube How to write simple Molecular Orbital Diagrams and determine the Bond order
Molecular orbital correlation diagrams for He2, He2+, N2 ... After a preliminary check with He2 and He2+, self‐consistent field calculations have been carried out for the nitrogen and carbon monoxide molecules and some of their positive ions for the range of internuclear distances from about 1.5 times equilibrium down to 0.01 bohr. To adequately describe the passage from the separated to the united atom limits, basis sets comprised of even‐tempered ...
gemmaron.nl Mar 16, 2022 · 101.3 hampton roads. WHPE Translator. 1s orbital wave function
(PDF) Inorganic Chemistry by Miessler ~ 5th Edition | Arnab ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry.
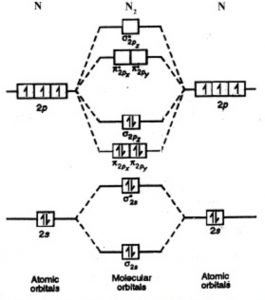
Molecular Orbital Theory - Purdue University Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Inorganic Chemistry 4th edition, Catherine ... - Academia.edu Inorganic Chemistry 4th edition, Catherine Housecroft. 2012. Thang Pham
PDF Miessler-Fischer-Tarr5e SM Ch 05 CM The S orbital energies are -22.7 eV (3s) and -11.6 eV (3p); the 1s of H has an energy of -13.6 eV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of
OneClass: construct the molecular orbital diagram for He2 ... construct the molecular orbital diagram for He2+2 and Sapling Learning Map d mcanoe Construct the molecular orbital diagram for Hez and then identify the bond order Bond order: 1s 1s D0.5 Ï . 1s 0 1.5 Atom Molecule Atom HeHe He Click within the blue boxes to add electrons O Next, Exit-
Construct the molecular orbital diagram for He2 and then ... Transcribed image text : Use the molecular orbital energy diagram below to answer the questions about bond order for the molecule He2 Number of Bonding Electrons Antibonding Electrons Number of He2 Bond Order This corresponds to A. Single bond...
Molecular Orbital Diagram For He2+ - schematron.org He2+ MO diagram. Eg: Li + H; Li has 1s + 2s, while H has 1s. This mix to form a sigma orbital from H1s+Li2s, a sigma* orbital and H1s-Li2s. The bond order of a simple molecule can be determined by looking at the number of electrons in bonding and antibonding molecular orbitals. Like electrons in.
Molecular Orbital Theory The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons - Number of Antibonding Electrons) = 1/2 x (2-2)=0.
Molecular Orbital Diagram For He2 A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 .















![Solved] The following is part of a molecular orbital energy ...](https://s3.amazonaws.com/si.question.images/images/question_images/1602/6/4/9/0105f867bb26575f1602649011138.jpg)




0 Response to "39 molecular orbital diagram for he2+"
Post a Comment