39 diagram of energy states and transitions in the hydrogen atom
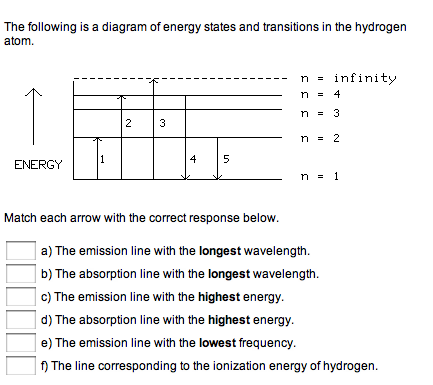
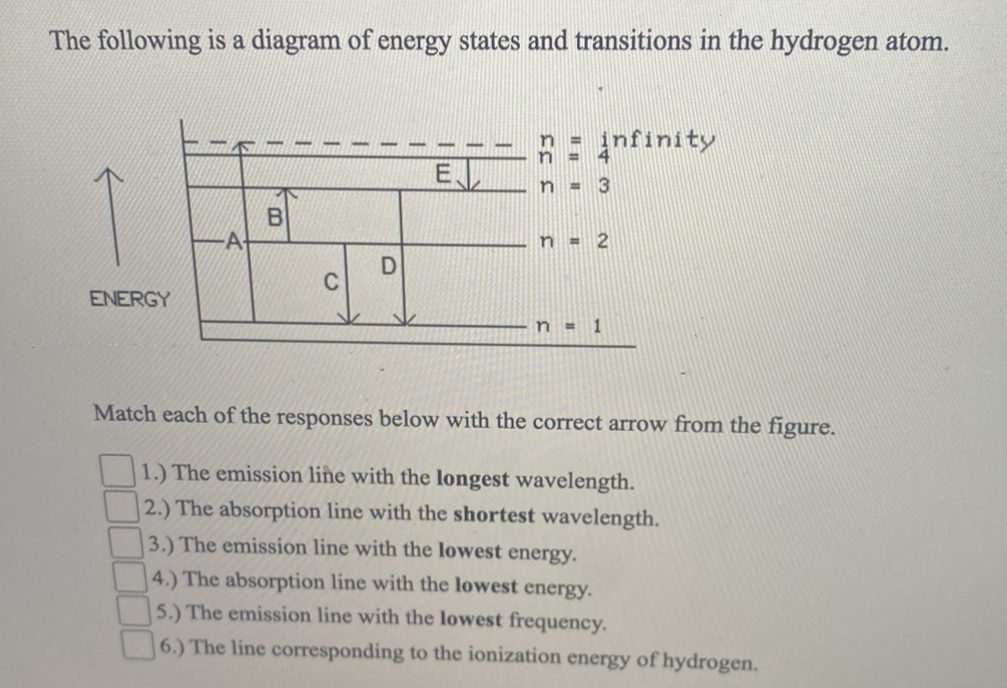
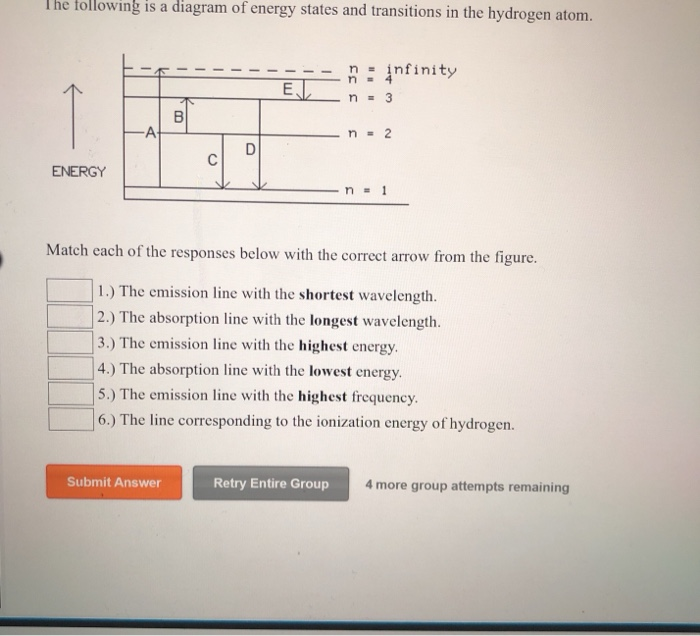
Transcribed image text: The following is a diagram of energy states and transitions in the hydrogen atom. Match each arrow with the correct response below. Question: The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from ...
Answer to: The following is a diagram of energy states and transitions in the hydrogen atom. By signing up, you'll get thousands of step-by-step...1 answer · Top answer: Emission spectra are observed when electrons return to the ground state from an excited state. Absorption spectra are observed when an electron goes ...

Diagram of energy states and transitions in the hydrogen atom
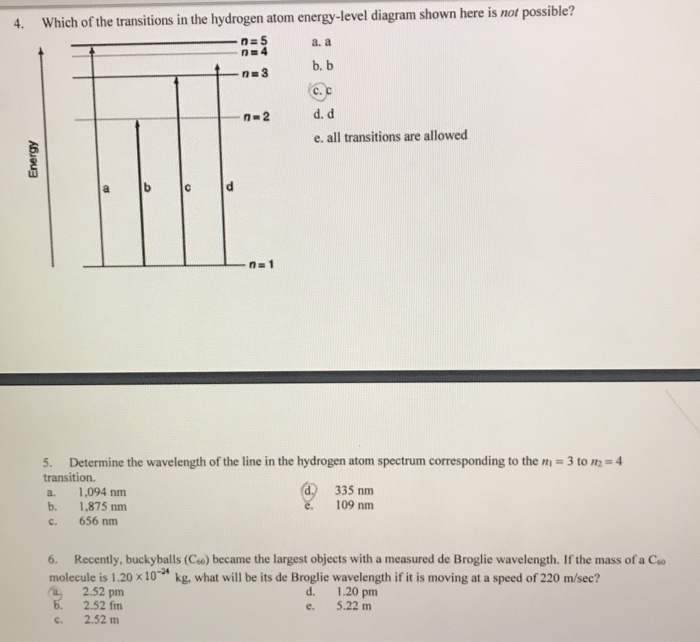
The following is a diagram of energy states and transitions in the hydrogen atom. Match each arrow with the correct response below. a) The emission line with the longest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest energy. d) The absorption line with the highest energy. e) The emission line with the highest frequency. Chemistry Q&A Library The following is a diagram of energy states and transitions in the hydrogen atom. n = infinity 4 n = 3 3 n = 2 ENERGY 4 n = 1 Match each arrow with the correct response below. a) The emission line with the shortest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest energy. Transcribed image text: The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the figure. a) The emission line with the longest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest energy.
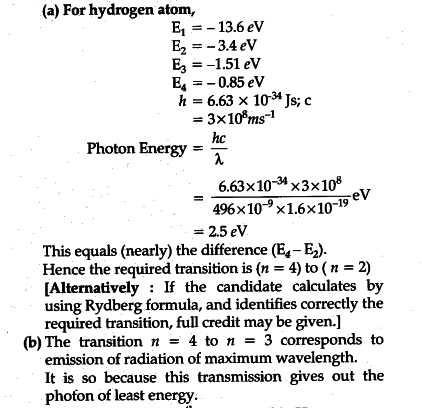
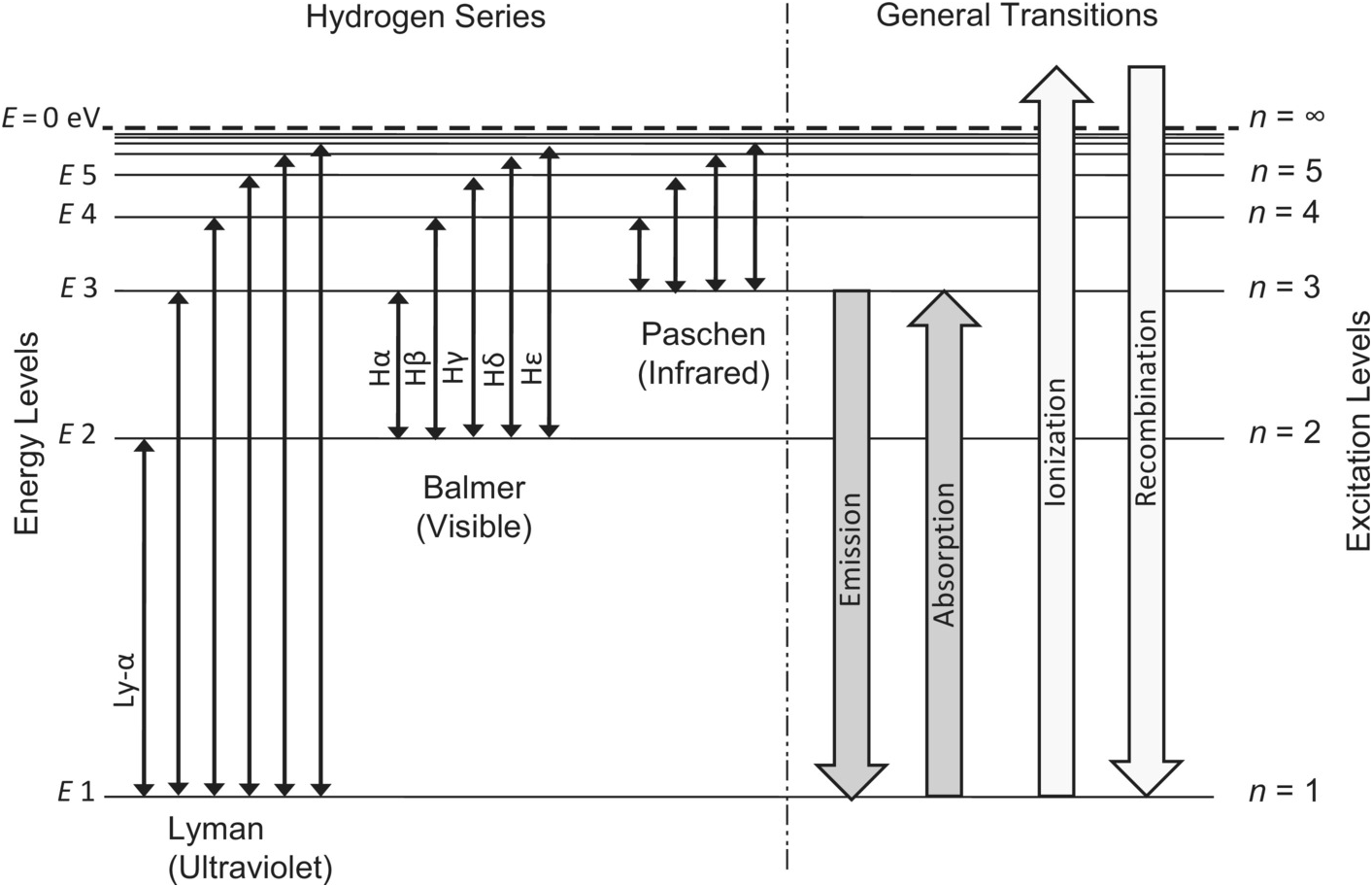
Diagram of energy states and transitions in the hydrogen atom. The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon. Chemistry questions and answers. The following is a diagram of energy states and transitions in the hydrogen atom. Match each arrow with the correct response below. The emission line with the longest wavelength. The absorption line with the longest wavelength. The emission line with the highest energy. The absorption line with the highest energy. Answer to: The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from ...1 answer · Top answer: We are asked to match the responses with the correct arrow.Emission is a transition process from a higher energy level to a lower energy level. Absorption ... The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the figure. a) The emission line with the shortest wavelength. b) The absorption line with the longest wavelength. c) The emission line with the highest energy.
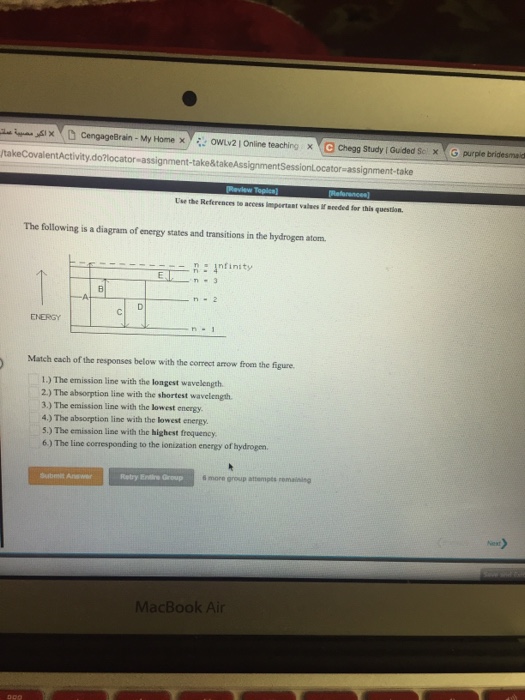
The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the figure. 1.) The emission line with the longest wavelength._____ 2.) The absorption line with the; Question: The following is a diagram of energy states and transitions in the hydrogen atom. Match ... Question: The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from ... Question: The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the figure. a) The emission line with the ... Chemistry Q&A Library The following is a diagram of energy states and transitions in the hydrogen atom. n = infinity n - 4 n = 3 3 n. = 2 1 ENERGY n = 1 Match each of the responses below with the correct arrow from the figure. a) The emission line with the shortest wavelength. The absorption line with the longest wavelength. c) The emission line with the lowest energy.
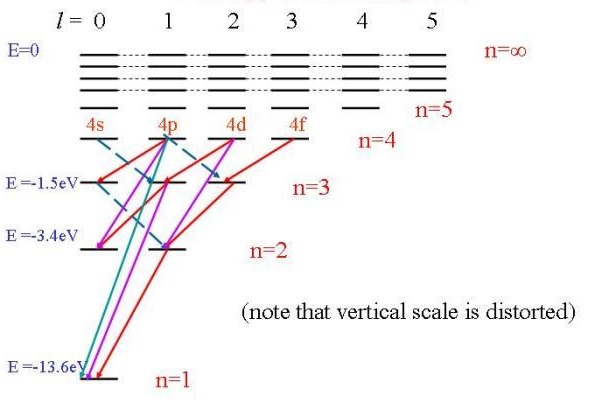
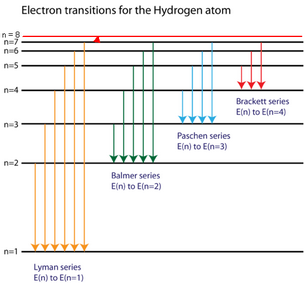
The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from a particular state to a lower state, it is losing energy. To conserve energy, a photon with an energy equal to the energy difference between the states will ... Transcribed image text: The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the figure. a) The emission line with the longest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest energy. Chemistry Q&A Library The following is a diagram of energy states and transitions in the hydrogen atom. n = infinity 4 n = 3 3 n = 2 ENERGY 4 n = 1 Match each arrow with the correct response below. a) The emission line with the shortest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest energy. The following is a diagram of energy states and transitions in the hydrogen atom. Match each arrow with the correct response below. a) The emission line with the longest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest energy. d) The absorption line with the highest energy. e) The emission line with the highest frequency.

Draw Energy Level Diagram For Hydrogen Atom Showing At Least Four Lowest Energy Levels Show The Transitions Responsible Physics Atoms 14302391 Meritnation Com

Draw A Partial Energy Level Diagram For Hydrogen All Wavelengths Are Ending At The N 2 State And The Energy Of The N 2 State Is 545 Aj Wavelengths Nm 411 26 434 77 487 10 658 42 Study Com
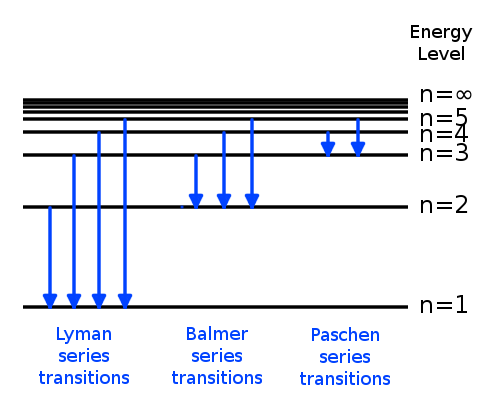
How Can An Electron Leap Between Atomic Levels Without Passing Through All The Space In Between Science Questions With Surprising Answers
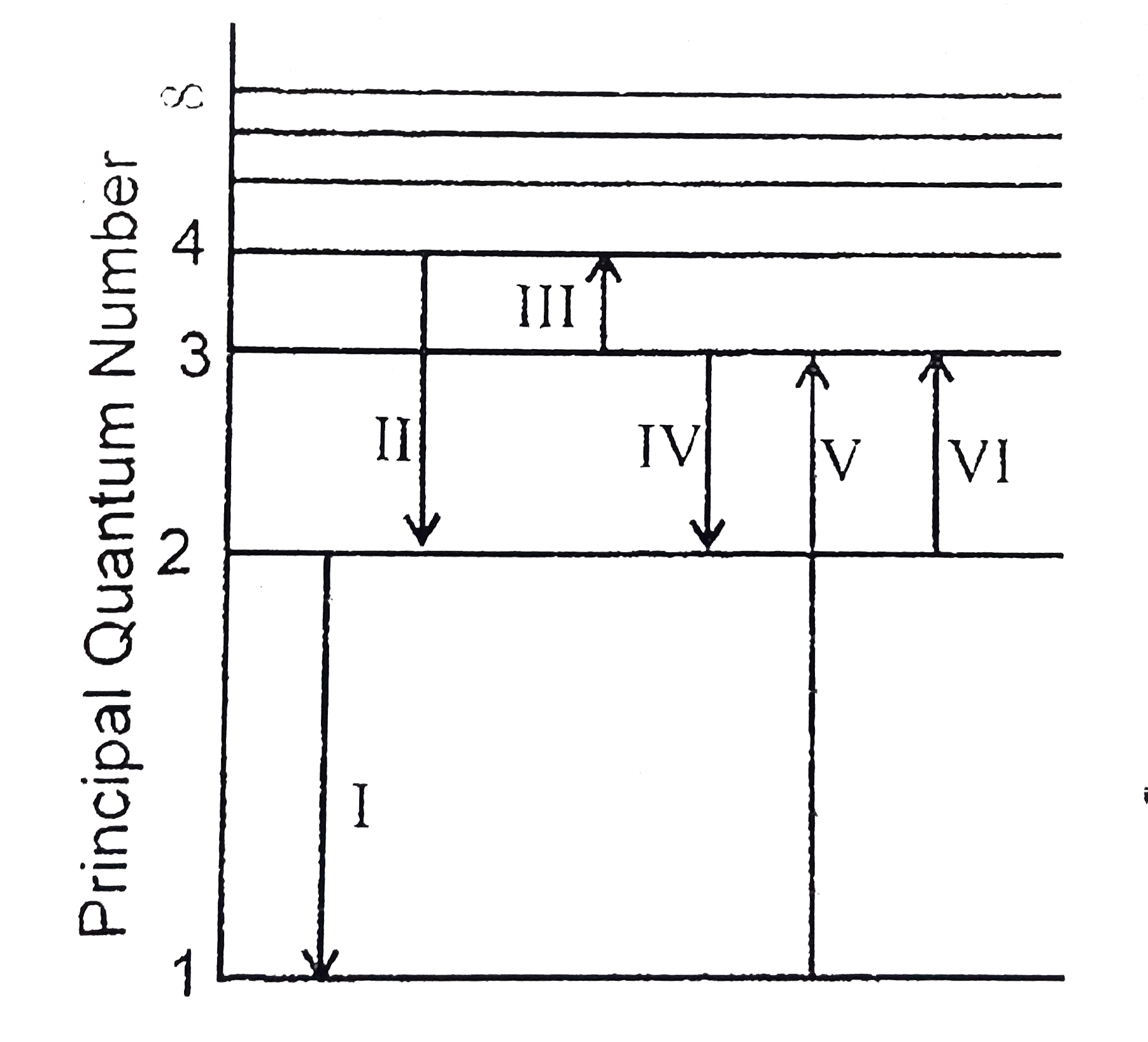
The Figure Shows An Energy Level Diagram For The Hydrogen Atom Several Transition Are Marked As I Ii Iii The Diagram Is Only Indicative And Not To Scale Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images
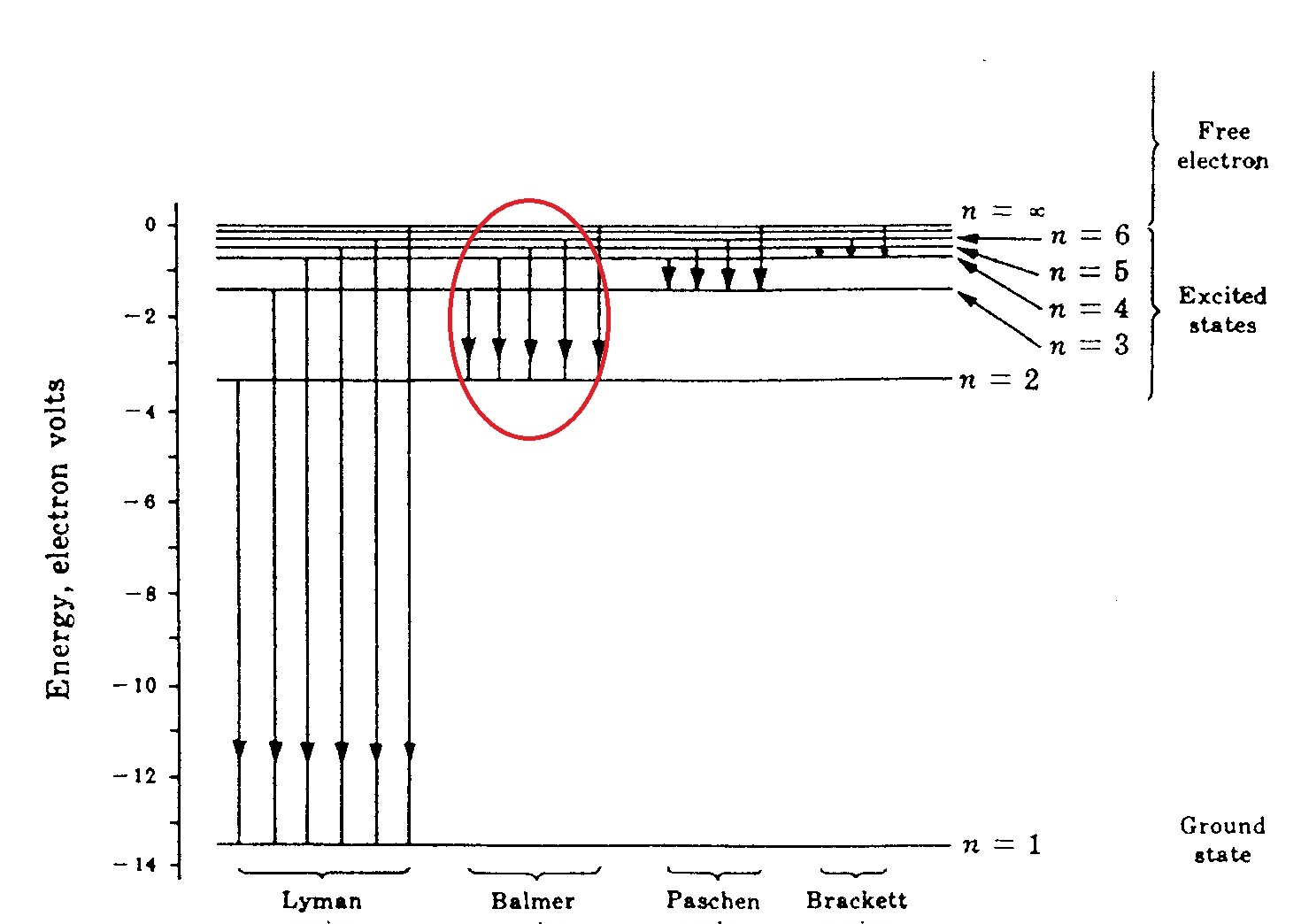
What Is The Wavelength In Nm Of A Photon Emitted During A Transition From The N 5 State To The N 2 State In The Hydrogen Atom Socratic
The Figure Shows Energy Level Diagram Of Hydrogen Atom I Find Out The Transition Which Results In The Emission Of A Photon Of Wavelength 496 Nm Sarthaks Econnect Largest Online Education Community

5 10 Draw Energy Level Diagram For Hydrogen Atom Showing At Least Four Lowest Energy Levels Show The Transitions Responsible For Emission Of Balmer Series 0 28 0 38 0 54 086 4 Energy Ev 1 5 1 3 W W Visible Visible 3 5 2 Balmer Series

Draw A Neat Labelled Energy Level Diagram For H Atom Showing The Transitions Explain The Series Of Spectral Lines For H Atom Whose Fixed Inner Orbit Numbers Are 3 And 4 Respectively

The Figure Shows An Energy Level Diagram For The Hydrogen Atom Several Transition Are Marked As I Ii Iii The Diagram Is Only Indicative And Not To Scale Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images
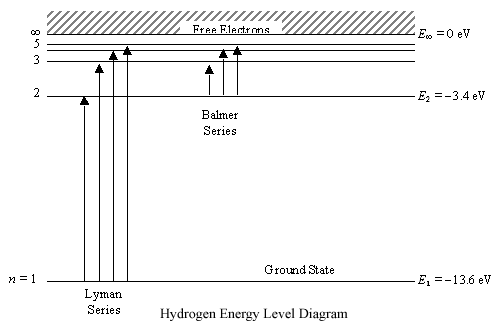











0 Response to "39 diagram of energy states and transitions in the hydrogen atom"
Post a Comment